Synthesis of bi- and tetradentate poly-Lewis acids by hydroalumination of poly-alkynyl-anthracene derivatives†
Abstract
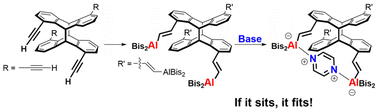
Photo-dimers of 1,5-diethynylanthracene and 1,5-bis[(trimethylsilyl)ethynyl]anthracene, obtained by UV radiation of the monomers, were treated with the bulky aluminium hydride [(SiMe3)2HC]2AlH in hydroalumination reactions. Hydroalumination of the tetraethynyl-substituted anthracene photo-dimers (syn and anti) led to fourfold aluminium-functionalized Janus-like products with two aluminium functions on each side oriented towards the same direction. The reactions of the monomeric anthracene derivatives with [(SiMe3)2HC]2AlH afforded semi-flexible bidentate Lewis acids, which are interesting building blocks for molecular chains bearing multiple Lewis-acidic functions. The aluminium-functionalized anti-photo-dimer was treated with various donor molecules to gain insight into its host–guest chemistry. All poly-Lewis acids and their adducts were characterized by X-ray diffraction experiments, multinuclear NMR spectroscopy and by elemental analyses.


 Please wait while we load your content...
Please wait while we load your content...