Open Access Article
Open Access ArticleDeaggregation properties and transmetalation studies of a zinc(II) salen-type Schiff-base complex†
Ivan Pietro
Oliveri
 ,
Giuseppe
Consiglio
,
Gabriella
Munzi
,
Giuseppe
Consiglio
,
Gabriella
Munzi
 ,
Salvatore
Failla
and
Santo
Di Bella
,
Salvatore
Failla
and
Santo
Di Bella
 *
*
Dipartimento di Scienze Chimiche, Università di Catania, I-95125 Catania, Italy. E-mail: sdibella@unict.it
First published on 14th July 2022
Abstract
This paper reports the synthesis and the deaggregation properties of a Lewis acidic Zn(II) salen-type Schiff-base complex derivative from diaminomaleonitrile and a systematic detailed study of its transmetalation with other metal ions in solution. In a solution of non-coordinating solvents, the complex is in a dimeric form, while in coordinating solvents or upon addition of a Lewis base it is stabilized as monomeric adducts. Experiments done in two solvents with different Lewis basicities indicate a major role of the stability of the starting adduct in transmetalation. Thus, using nitrate or perchlorate salts, acetonitrile solutions of the complex give an immediate and complete transmetalation with Cu2+, while with Co2+ and Ni2+ a much slower transmetalation rate is observed. Instead, using chloride salts a fast and complete transmetalation is observed for divalent ions of the first transition series (Mn2+, Fe2+, Co2+, Ni2+, Cu2+), indicating the role of the chloride in stabilizing the transition state of the transmetalation. On the other hand, DMF solutions of the complex are less prone to transmetalation, according with the greater basicity of the solvent and, hence, the greater stability of the related adducts with the complex. Therefore, the nature of the solvent and the counteranion allow controlling the transmetalation process of this Zn(II) Schiff-base complex.
1. Introduction
Transition metal-based molecular materials are currently widely investigated for their functional properties.1–5 Among them, zinc(II) complexes of Schiff-base ligands represent a promising class of materials with diverse optical and optoelectronic properties.6–12 An interesting family is constituted by Lewis acidic Zn(salen)-type complexes, whose optical properties are mainly related to the absence/presence of a Lewis base.13,14 In the first case, these complexes tend to dimerize/oligomerize in order to saturate their coordination sphere, while in the presence of Lewis bases they form monomeric adducts.15–17 Aggregate species or monomeric adducts are characterized by very different optical spectroscopic properties, useful for using these complexes as chemosensors of Lewis bases.13,14,18The stability of the Zn(salen)·Lewis base adducts is certainly an important feature for the chemistry of these complexes, mainly related to the Lewis basicity of the coordinated species.19,20 Therefore, the different stabilities of the adducts are expected to affect other properties of these complexes. One of these could be the ability of the Zn(II) ion to transmetalate with main group and/or transition metal ions. In this regard, few examples have been reported in the literature involving transmetalation of Zn(salen)-type complexes.21–23 Transmetalation, or metal exchange, represents an unconventional approach in coordination and organometallic chemistry for its use in synthesis, catalysis, and sensing.24–29
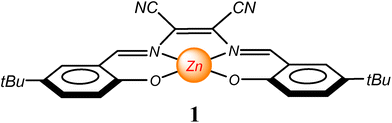
Among Zn(salen)-type complexes, colourful derivatives from the 2,3-diaminomaleonitrile, Zn(salmal), are certainly those more interesting for their optical absorption and fluorescence emission properties upon deaggregation.30–37 In order to further explore these varied characteristics, we have chosen a Zn(salmal) complex, 1, having different substituents on the salicylidene rings. In particular, we found that the presence of 5-tert-butyl-bulky substituents makes this complex sufficiently soluble in a range of coordinating/non-coordinating solvents, giving us the possibility to explore its spectroscopic properties in solution, even in comparison to previous studies on amphiphilic Zn(salmal) complexes with longer alkyl substituents.30 Moreover, in this work, a study on the transmetalation ability of this complex towards other metal ions is reported, in relation to the nature of the starting adduct. This represents for the first time a systematic transmetalation study in solution for this family of complexes.
2. Results and discussion
2.1. Synthesis and characterization
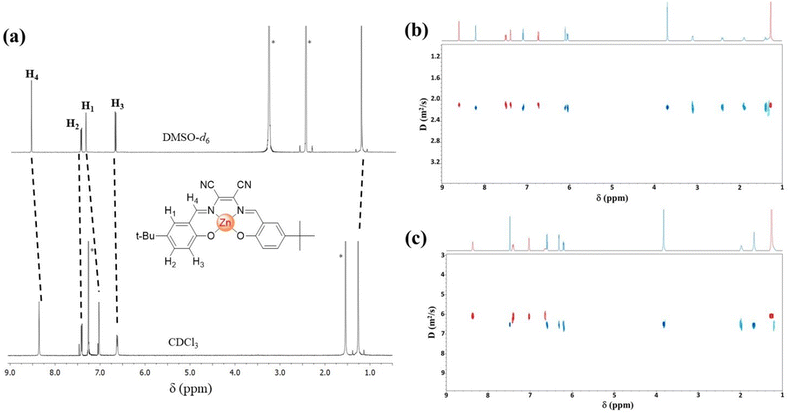
The synthetic procedure for the preparation of complex 1 involves the reaction of 5-t-butyl-2-hydroxybenzaldehyde and 2,3-diaminomaleonitrile with Zn(ClO4)2 in the presence of a base. The isolated compound is moderately soluble in the non-coordinating chloroform and other low-polar solvents and soluble in polar coordinating solvents, such as dimethylsulfoxide (DMSO), dimethylformamide (DMF), and acetonitrile (MeCN).The 1H NMR spectrum of 1 in DMSO-d6 shows that the expected signals are consistent with its molecular structure. On switching to the spectrum in CDCl3, a substantial up-field shift of the signals relative to H1 (0.36 ppm) and H4 (0.25 ppm) and a slight up-field shift of the signals relative to H2 (0.08 ppm) and H3 (0.11 ppm) protons are observed, in addition to a slight broadening of the H3 and H4 peaks (Fig. 1a). As previously reported,30 these significant shifts on passing from DMSO-d6 to CDCl3 are consistent with the formation of dimeric aggregates in a solution of the non-coordinating CDCl3 and the existence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts, 1·DMSO-d6, in the coordinating DMSO-d6 solvent.
1 adducts, 1·DMSO-d6, in the coordinating DMSO-d6 solvent.
Diffusion-ordered NMR spectroscopy (DOSY) measurements further support this hypothesis. DOSY experiments were performed in the presence of a known reference compound.34 In particular, the Schiff-base Zn(II) complex derivative of trans-1,2-cyclopentanediamine38 was used for DOSY measurements of 1 in CDCl3 (Fig. 1c). These indicate the presence of a single component in the diffusion dimension (D = 6.05 × 10−10 m2 s−1), having a comparable diffusion coefficient of the reference compound (D = 6.55 × 10−10 m2 s−1), with an estimated molecular mass of 1012 Da, in agreement with the calculated molecular mass (983.8 Da) for the dimer of 1. DOSY measurements of 1 in DMSO-d6 were performed in the presence of the Zn(II) complex derivative from trans-1,2-diaminocyclohexane39 (Fig. 1b). A diffusion coefficient value of 2.08 × 10−10 m2 s−1 (D = 2.16 × 10−10 m2 s−1 for the reference compound) and an estimated mass of 571 Da, in agreement with the formation of an adduct with the solvent (1·DMSO-d6 = 574 Da), are found, thus confirming the existence of monomeric species in solution of coordinating solvents.
2.2. Optical spectroscopic properties in solution and deaggregation experiments
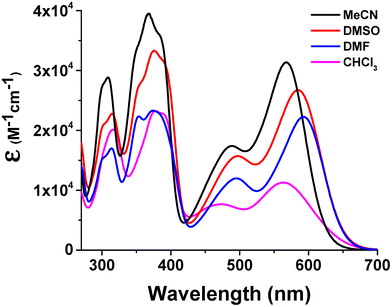
The UV/vis optical absorption spectrum of 1 in CHCl3 shows two bands in the UV region (λmax = 316 nm and 382 nm) and two bands with lower intensity in the visible region, centred at λmax = 473 nm and 570 nm (Fig. 2). On changing to coordinating solvents (Fig. 2), a general hyperchromism of the absorption bands, more pronounced for the longer wavelength band, is observed which is also red-shifted (λmax = 580 nm in DMF), accompanied by an evident naked-eye colour change of the solution from red-wine to blue-violet (in DMF).These experimental findings are consistent with the presence of aggregate species in solution of non-coordinating solvents and monomeric adducts with the solvent axially coordinated in coordinating solvents, as previously found for analogous Zn(salmal) derivatives.30,31 In fact, starting from solutions of 1 in non-coordinating solvents, the addition of a Lewis base leads to deaggregation with the formation of monomeric adducts having optical characteristics analogous to those observed in solution of coordinating solvents. Deaggregation can be accomplished using various Lewis bases, such as primary, secondary, and tertiary amines, aromatic amines, or ethers. Spectrophotometric titrations of CHCl3 solutions of 1 with pyridine (Fig. 3) and isopropylamine (Fig. S1†) are reported as representative examples. In all instances, the addition of the Lewis base involves the formation of a new, strong absorption band at longer wavelengths (λmax = 595 nm, for pyridine) responsible for the dramatic colour change of the solution (from red-wine to indigo). The curve fit analysis of the binding isotherm at 595 nm as a function of the concentration of pyridine added allowed the estimation of the binding constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K value of 5.45) for the formation of the 1·py adduct.
K value of 5.45) for the formation of the 1·py adduct.
Pyridine has previously been used as a reference Lewis base to deaggregate a series of Zn(salen)-type complexes in non-coordinating solvents to rank their Lewis acidic character within the series.20 Thus, the derived binding constant for the formation of the 1·py adduct allows establishing the Lewis acidity of 1 in comparison to previously investigated complexes. In particular, a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K value of 5.45 indicates a slightly weaker Lewis acidic character of 1 in comparison to that estimated for the related Zn(salmal) complex having 4-alkoxy substituents in the salicylidene rings.20
K value of 5.45 indicates a slightly weaker Lewis acidic character of 1 in comparison to that estimated for the related Zn(salmal) complex having 4-alkoxy substituents in the salicylidene rings.20
2.3. Transmetalation studies
In order to explore the transmetalation ability of 1 towards several metal cations in relation to the stability of the starting adduct, two different solvents, MeCN and DMF, were considered. Both of them are coordinating solvents, but have different Lewis basicities.17 Thus, different stabilities of the related adducts can be anticipated. In fact, the derived binding constants for the formation of the adducts, evaluated from spectrophotometric titrations upon deaggregation of 1 in CHCl3 (Fig. S2 and S3†),20 indicate for the 1·DMF adduct a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K value of 2.95, more than two orders of magnitude larger than that for the 1·MeCN adduct (log
K value of 2.95, more than two orders of magnitude larger than that for the 1·MeCN adduct (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 0.88). Therefore, a different transmetalation behaviour of 1 is expected in these coordinating solvents.
K = 0.88). Therefore, a different transmetalation behaviour of 1 is expected in these coordinating solvents.
Initially, nitrate salts of most common divalent and some trivalent cations were considered. The occurrence of transmetalation was first simply checked by optical absorption measurements immediately after the addition of stoichiometric amounts of aqueous solutions of nitrate salts to MeCN solutions of 1. Except for Fe3+, Al3+, and Hg2+ ions, which lead to the demetalation of 1 (vide infra), sizable changes in the optical absorption spectra of 1, consistent with transmetalation, are observed only in the case of the addition of Cu(NO3)2, while the addition of all other cations involves small or negligible changes. In particular, the equimolar addition of Cu(NO3)2 to 1 causes a decrease in the longer wavelength absorption band at λmax = 567 nm and the appearance of a new optical absorption feature at shorter wavelengths (Fig. 4). Upon addition of a 2-fold molar excess of Cu(NO3)2, the disappearance of the band at λmax = 567 nm and the definition of a new optical absorption band centred at λmax = 524 nm are observed, accompanied by a colour change of the solution from violet to magenta. These optical absorption changes upon addition of Cu(II) to MeCN solutions of 1 are in agreement with transmetalation. In fact, identical optical spectroscopic features are obtained from MeCN solutions of the synthesized and purified Cu(salmal) complex (Fig. 4). 1H NMR titrations of 1 in CD3CN, upon successive addition of a Cu(NO3)2 solution in D2O, indicate an increasing broadening of the proton signals, consistent with the transmetalation of the Zn2+ ion with the paramagnetic Cu2+ ion.40–42 A complete disappearance of proton signals in the 4–9 ppm region is observed after the addition of a 2-fold molar excess of a Cu(NO3)2 solution (Fig. S4†).
Transmetalated solutions of Cu(salmal) are stable, as no optical absorption changes are observed even after several weeks. Moreover, analogous results are obtained using Cu(ClO4)2 as the source of Cu2+ ions. Therefore, the addition of Cu2+ ions, either as nitrate or perchlorate, to MeCN solutions of 1 results in a fast and complete transmetalation, thus indicating that the Cu(salmal) complex is thermodynamically more stable than the Zn(II) analogue and the counteranion does not affect transmetalation. Transmetalation with Cu(II) has been reported in the literature also for other families of Zn(II) complexes.26,43–45
The observed dramatic variations in the optical absorption spectra upon the addition of a 2-fold molar excess of Fe3+, Al3+, and Hg2+ ions, as nitrate salts (Fig. S5†), with the almost disappearance of the longer wavelength band and the formation of a new strong optical absorption band at λmax = 460 nm, accompanied by a colour fading from violet to orange-yellow, cannot be ascribed to transmetalation. Rather, a demetalation process likely occurs, as a consequence of the acid hydrolysis of ferric and46 aluminium salts47 and Hg(II) nitrate.48 To support this hypothesis, spectrophotometric titrations of MeCN solutions of 1 with aqueous perchloric acid were performed (Fig. S6†). Identical optical absorption spectral variations, such as those observed by addition of Fe(III), Al(III), and Hg(II) to MeCN solutions of 1, are obtained. A complete demetalation of 1 occurs upon the addition of a 2.5-fold molar excess of the perchloric acid.
The transmetallation kinetics of the MeCN solutions of 1 upon the addition of 2-fold molar excess of the involved nitrate salts, left at room temperature, was further monitored by recording over time the optical absorption spectra. Except for Co2+ and Ni2+, no further appreciable changes, as compared to the measurements recorded immediately after the addition of various cations, were observed even after a week. In the case of Co2+, a progressive hypochromism and broadening of the absorption bands at longer wavelengths of 1 are observed within 24 hours, suggestive of a transmetalation (Fig. 5). The 1H NMR spectrum of 1 in CD3CN, recorded 24 hours after the addition of 2-fold molar excess of Co(NO3)2 in D2O, shows sharp, shifted signals with respect to those of 1 (Fig. 6), indicating the formation of a low-spin diamagnetic Co(III) complex upon aerobic oxidation of the Co2+ ion.49–53 In particular, resonances of the imine CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (8.20 ppm) and aromatic (7.2–7.7 ppm) protons fall within the range of those found in other octahedral Co(III) salen-type complexes, axially coordinated with two Lewis bases.54,55 Instead, for Ni2+ a slow and progressive transmetallation is observed within seven days. The complete transmetalation of 1 with Ni2+ is demonstrated by the comparison of the optical absorption spectrum recorded after seven days, which is identical to that recorded for the synthesized Ni(salmal) complex (Fig. 7). Perchlorate salts of Co(II) and Ni(II) give identical results. Interestingly, transmetalation experiments using Ni(II) nitrate and heating the solution at 60 °C, under stirring, lead to a complete transmetalation after 3 hours (Fig. S7†), instead of seven days at room temperature. Therefore, in this case, the temperature dramatically affects the transmetallation kinetics.
N (8.20 ppm) and aromatic (7.2–7.7 ppm) protons fall within the range of those found in other octahedral Co(III) salen-type complexes, axially coordinated with two Lewis bases.54,55 Instead, for Ni2+ a slow and progressive transmetallation is observed within seven days. The complete transmetalation of 1 with Ni2+ is demonstrated by the comparison of the optical absorption spectrum recorded after seven days, which is identical to that recorded for the synthesized Ni(salmal) complex (Fig. 7). Perchlorate salts of Co(II) and Ni(II) give identical results. Interestingly, transmetalation experiments using Ni(II) nitrate and heating the solution at 60 °C, under stirring, lead to a complete transmetalation after 3 hours (Fig. S7†), instead of seven days at room temperature. Therefore, in this case, the temperature dramatically affects the transmetallation kinetics.
 | ||
| Fig. 6 Comparison of 1H NMR spectra of 1 (1.0 × 10−4 M) in CD3CN before (top) and after the addition of 2-fold molar excess of a D2O solution of Co(NO3)2 recorded after 24 hours (down). | ||
The possible effect of other counteranions on transmetalation was also investigated. Surprisingly, the addition of stoichiometric amounts of metal chlorides to MeCN solutions of 1 lead to very different results from those previously obtained for nitrate or perchlorate salts (Fig. 8). In fact, optical absorption measurements recorded immediately after the addition of 2-fold molar excess of various chlorides (30 min for Co(II) and Ni(II)) indicate substantial spectral changes for all the involved divalent metal ions of the first transition series, including Mg(II), consistent with transmetallation. These absorption spectral changes are in agreement with those found for the homologous series of M(salen)-type complexes.21,56,57
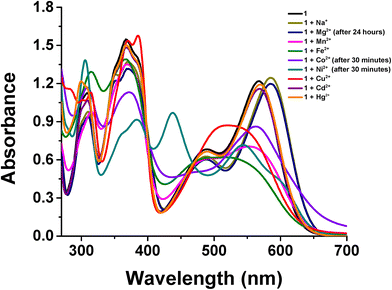 | ||
| Fig. 8 Optical absorption spectra of 1 (40.0 μM solution in MeCN) before and after the addition of 2-fold molar excess of several cations (as aqueous solutions of chloride salts). | ||
The ESI-MS analysis of the transmetalated MeCN solutions after the addition of a 2-fold molar excess of various metal chlorides indicates the presence of a molecular peak for M(salmal) (M = Mg, Mn, Fe, Co, Ni, Cu) complexes. In particular, the ESI-mass spectra of the transmetalated solutions with Mn(II), Fe(II), and Co(II) show an intense peak related to the [M(salmal)]+ ion (Fig. S8†). Instead, those with Mg(II), Ni(II), and Cu(II) show the presence of the [M + H]+ molecular ion (Fig. S9†). Moreover, the optical absorption measurements of these transmetalated solutions with Cu2+, Co2+, and Ni2+ show identical spectra to those recorded using the nitrate or perchlorate salts of Cu(II), Co(II), and Ni(II), although transmetalation for the latter two ions took place in a much longer time. 1H NMR measurements of the transmetalated CD3CN solutions of 1, recorded after the addition of a 2-fold molar excess of NiCl2 in D2O, show a spectrum identical to that recorded for the Ni(salmal) complex in CD3CN solution (Fig. S10†). 1H NMR titrations of 1 in CD3CN, after the successive addition of CoCl2 in D2O, show the appearance in the 1H NMR spectrum of a new distinct set of sharp signals, strongly shifted with respect to those of 1 (Fig. S11†) and identical to those observed upon the addition of Co(NO3)2 to 1. No further changes in the 1H NMR spectra are observed after the addition of 2-fold molar excess of CoCl2. DOSY experiments, performed after the addition of a half-molar amount of CoCl2 to a CD3CN solution of 1, indicate the presence of a single component in the diffusion dimension (D = 11.6 × 10−10 m2 s−1), having a diffusion coefficient comparable to that of the 1·CD3CN adduct used as the reference species (D = 12.3 × 10−10 m2 s−1). These data are consistent with the presence of a monomeric Co(III) complex in solution. The identical chemical shift of signals found either upon transmetalation with perchlorate or chloride salts (Fig. S12†) suggests that the anions are out of the coordination sphere of the Co(III) complex. Therefore, the overall data suggest that after the transmetalation of 1 with the Co2+ ion, the complex evolves towards the formation of a low-spin diamagnetic [Co(salmal)]+ species, whose hexacoordination is fulfilled by the coordination of the solvent.58,59 The 1H NMR spectra of transmetalated solutions with Mn2+, recorded immediately after the addition of 2-fold molar excess of MnCl2, show the complete disappearance of all proton signals, according with the formation of a paramagnetic complex.40 While transmetalated solutions of 1 with Cu2+, Ni2+, and Co2+ (oxidized to Co3+) are stable over time, those transmetalated with Fe2+ completely decompose within 30 min and those with Mn2+ gradually decompose within a few days.
In the case of Mg2+, the transmetalated Mg(salmal) complex initially formed successively evolves (within 24 hours) towards the formation of the adduct (Fig. S13†). Analogously, the addition of Na(I) chloride to MeCN solutions of 1 leads to the formation of chloride adducts by solvent displacement with the chloride ion.14,60–63 Indeed, spectrophotometric titrations of 1 in MeCN with an aqueous solution of tetrabutylammonium chloride (TBACl) show analogous optical absorption changes as those observed upon addition of Mg(II) and Na(I) chlorides, indicating the formation of stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1·Cl−TBA+ adducts with a saturation point after the addition of ca. 1.6-fold molar excess of TBACl (Fig. S14†). Finally, the addition of the remaining Cd(II) and Hg(II) chlorides to 1 does not involve any optical absorption change, presumably because of their presence in the molecular form in solution.64,65
1 1·Cl−TBA+ adducts with a saturation point after the addition of ca. 1.6-fold molar excess of TBACl (Fig. S14†). Finally, the addition of the remaining Cd(II) and Hg(II) chlorides to 1 does not involve any optical absorption change, presumably because of their presence in the molecular form in solution.64,65
In order to assess the role of the chloride ion in the transmetalation process, further experiments were done. For this study, we have chosen the Ni2+ ions because with tetradentate ligands it gives stable square planar complexes, without the need for any auxiliary ligands/solvent.66–68 Therefore, the stability of the transmetalated Ni(salmal) complex will be not affected by the nature of the counteranion.
Starting from a MeCN solution of 1, the addition of a 2-fold molar excess of Ni(ClO4)2 followed by the addition of an equimolar amount (with respect to perchlorate ions) of chloride ions, as NaCl, leads to a complete transmetalation within 30 min, as that obtained using NiCl2 (Fig. S15†). The same occurs also in the presence of a stoichiometric excess of perchlorate anions added as TBAClO4. Identical results are obtained upon adding NaCl to 1 before the addition of Ni(ClO4)2. Moreover, if one-half molar amount of NaCl is added to 1, a complete transmetallation occurs in a much longer time (ca. 2 days). It then turns out that the chloride anion plays a role in stabilizing the transition state of the transmetalation (Scheme 1).
In order to investigate the transmetalation process starting from a different adduct, 1·DMF, analogous experiments were performed using solutions of the complex 1 in DMF. The addition of 2-fold molar excess of either nitrate or perchlorate of the involved cations does not produce any appreciable change in the optical absorption spectra measured immediately after the addition of the cations, except for some small variations for Co2+ and Cu2+ (Fig. S16†). A complete transmetalation of 1 with Cu2+ occurs after ca. 20 hours (Fig. S17†), in striking contrast to the immediate transmetalation observed in MeCN.
On the other hand, the addition of metal chlorides to DMF solutions of 1 again leads to very different results than those observed in MeCN. An almost complete transmetalation (within 5 min) occurs only in the case of the addition of CuCl2 (Fig. S18†). Instead, the addition of the other chlorides (Na+, Mg2+, Mn2+, Co2+, and Ni2+) gives absorption changes analogous to those observed upon titration of 1 in DMF with TBACl (Fig. S19†), thus indicating the formation of 1·chloride adducts. However, for Co(II) and Ni(II), the chloride adducts initially formed successively evolve, at different times, into the transmetalated species. In the case of Co(II), transmetalation is observed after 5 hours, while for Ni(II) a complete transmetalation occurs after ca. 50 hours (Fig. S20†). Instead, for Mn(II) a subsequent gradual decomposition is observed. Analogously, as observed for MeCN solutions of 1, the addition of HgCl2 and CdCl2 to DMF solutions of 1 does not involve any absorption change. Finally, the addition of FeCl2 to DMF solutions of 1 leads first to demetalation and then to decomposition. Titrations of DMF solutions of 1 with perchloric acid indicate a complete demetalation upon the addition of a 3.75-fold molar excess of acid (Fig. S21†). In comparison with the demetalation of 1 in MeCN, this is in agreement with the greater stability of the 1·DMF adduct. The transmetalation behaviour of 1 in MeCN and DMF towards the involved metal cations is summarized in Table 1.
| Solvent | Counteranion | Na+ | Mg2+ | Mn2+ | Fe2+ | Co2+ | Ni2+ | Cu2+ | Cd2+ | Hg2+ | Pb2+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a nt = no transmetalation; t0 = transmetalation immediately after the addition of the metal salt; dm = demetalation; ad = 1·chloride adduct formation. b Subsequent adduct formation. c Subsequent decomposition. | |||||||||||
| MeCN | Nitrate/perchlorate | nt | nt | nt | nt | 24 h | 7 days | t 0 | nt | dm | nt |
| Chloride | ad | t 0 | t 0 | t 0 | 30 min | 30 min | t 0 | nt | nt | — | |
| DMF | Nitrate/perchlorate | nt | nt | nt | nt | 30 h | 100 h | 20 h | nt | dm | nt |
| Chloride | ad | ad | adc | dmc | 5 h | 50 h | 5 min | nt | nt | — | |
In summary, DMF solutions of 1 are less prone to transmetalation than MeCN solutions, and this different behaviour can be related to the greater stability of the 1·DMF adduct, which is consequently reflected by the slower transmetalation kinetics.
3. Conclusions
This paper reports the synthesis and the deaggregation properties of a Lewis acidic Zn(salen)-type complex, and a systematic study on the transmetalation in solution with other metal ions. Although the transmetalation process is thermodynamically driven, its kinetics is strongly affected by the stability of the 1·solvent adducts and the nature of the counteranion of the incoming cation. While MeCN is a solvent with a weak Lewis basicity, yielding adducts suitable for a facile transmetalation, DMF possessing a stronger Lewis basicity forms more stable adducts and is less prone to transmetalation. Moreover, the presence of the chloride anion significantly accelerates the kinetics of the process in MeCN solutions, with a fast and complete transmetalation for divalent ions of the first transition series, indicating a major involvement of the chloride ion in the transmetalation mechanism. Therefore, the nature of the solvent and the counteranion allow controlling the transmetalation process of this Zn(salmal) complex. In conclusion, the interesting transmetalation results emerging from this study certainly deserve further investigations within the family of Zn(salen)-type Schiff-base complexes as these findings can be exploited for the straightforward synthesis of new M(salen)-type complexes and for sensing specific cations.4. Experimental
4.1. Materials and general procedures
All the chemicals used were purchased from Sigma-Aldrich (Merck) and used as received. Zinc perchlorate hexahydrate was used for the template synthesis of complex 1. Caution: Perchlorate salts of metal compounds in the presence of organics are potentially explosive. Only small amounts of the material should be cautiously handled. Chloroform stabilized with amylene was used for spectrophotometric measurements. It was purified as follows before use: left overnight in anhydrous K2CO3, filtered and stored over molecular sieves (3 Å) in the dark under an argon atmosphere. DMF was dried over molecular sieves (3 Å). CDCl3 was stored over molecular sieves (3 Å), while all the other deuterated solvents were used as received. All reactions were performed under an inert atmosphere of nitrogen.4.2. Physical measurements
Elemental analyses were performed on a Carlo Erba 1106 elemental analyzer. ESI-MS spectra were recorded on a Thermo Fisher API 2000 mass spectrometer. ESI-MS spectra were achieved using MeCN solutions of complex 1 (40 μM) immediately after the addition of aqueous solutions of the chloride salts, except for Ni(II) and Co(II), for which ESI-MS spectra were achieved 30 min after the addition of NiCl2 and CoCl2. 1H NMR experiments were performed at 27 °C on a Varian Unity S 500 (499.88 MHz for 1H) spectrometer. Tetramethylsilane (TMS) has been used as the internal reference for all NMR experiments. 1H DOSY measurements in CDCl3 were performed using the ZnII complex derivative of the trans-1,2-diaminocyclopentane as the reference species (double helicate Zn2L2 structure, 863.6 Da),38 while the ZnII complex derivative of the trans-1,2-diaminocyclohexane was used as the reference for DOSY measurements in DMSO-d6 (adduct with DMSO, 530 Da).39 Further experimental details are reported elsewhere.34,38,39 UV/vis optical absorption spectra were recorded at room temperature using an Agilent Cary 60 spectrophotometer.4.3. Spectrophotometric titrations and transmetalation experiments
Solutions of Lewis bases and metal salts were added to solutions of the complex 1 using Rainin (METTLER TOLEDO) positive displacements pipettes. Titrations were performed using a 1 cm path length cuvette. At least three replicate titrations were performed for each Lewis base. Experimental details of the determination of binding constants are reported elsewhere.60 Transmetalation experiments were performed at room temperature, without stirring, and monitored immediately after the addition of aqueous metal salt solutions and over time. Aqueous solutions of metal salts were obtained by dissolving salts in distilled water and used as prepared. The addition of distilled water or D2O to MeCN solutions of 1 does not involve any change in the optical absorption nor 1H NMR spectra, indicating that no demetalation/decomposition occurs.4.4 Syntheses
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.49 (dd, 3JHH = 9 Hz, 4JHH = 3 Hz, 2H; ArH), 7.39 (d, 3JHH = 9 Hz, 1H; ArH), 6.76 (d, 3JHH = 9 Hz, 2H; ArH), 1.26 (s, 18H, C(CH3)3). 13C NMR (125 MHz, DMSO-d6): δ = 31.39, 34.03, 112.26, 118.20, 121.97, 124.41, 131.82, 136.12, 136.97, 164.20, 173.55.
N), 7.49 (dd, 3JHH = 9 Hz, 4JHH = 3 Hz, 2H; ArH), 7.39 (d, 3JHH = 9 Hz, 1H; ArH), 6.76 (d, 3JHH = 9 Hz, 2H; ArH), 1.26 (s, 18H, C(CH3)3). 13C NMR (125 MHz, DMSO-d6): δ = 31.39, 34.03, 112.26, 118.20, 121.97, 124.41, 131.82, 136.12, 136.97, 164.20, 173.55.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.63 (dd, 3JHH = 9 Hz, 4JHH = 3 Hz, 2H; ArH), 7.56 (d, 3JHH = 3 Hz, 1H; ArH), 7.04 (d, 3JHH = 9 Hz, 2H; ArH), 1.30 (s, 18H, C(CH3)3).
N), 7.63 (dd, 3JHH = 9 Hz, 4JHH = 3 Hz, 2H; ArH), 7.56 (d, 3JHH = 3 Hz, 1H; ArH), 7.04 (d, 3JHH = 9 Hz, 2H; ArH), 1.30 (s, 18H, C(CH3)3).
13C NMR (125 MHz, CDCl3): δ = 30.82, 34.07, 77.00, 77.25, 109.29, 118.85, 121.97, 122.12, 122.65, 127.56, 137.39, 140.80, 155.78, 168.10.
Conflicts of interest
There are no conflicts of interest to be declared.Acknowledgements
This research was supported by the University of Catania, PIACERI 2020/2022, Linee di Intervento 2 e 3.References
- M. Atzori and F. Artizzu, Functional Molecular Materials: An Introductory Textbook, Pan Stanford Publishing, 2018 Search PubMed.
- C. Wang, D. Liu and W. Lin, J. Am. Chem. Soc., 2013, 135, 13222–13234 CrossRef CAS PubMed.
- I. Ratera and J. Veciana, Chem. Soc. Rev., 2012, 41, 303–349 RSC.
- Molecular Materials, ed. D. W. Bruce, D. O'Hare and R. I. Walton, John Wiley & Sons, 2010 Search PubMed.
- V. W.-W. Yam, Acc. Chem. Res., 2002, 35, 555–563 CrossRef CAS PubMed.
- R. Diana and B. Panunzi, Molecules, 2020, 25, 4984 CrossRef CAS PubMed.
- A. Terenzi, A. Lauria, A. M. Almerico and G. Barone, Dalton Trans., 2015, 44, 3527–3535 RSC.
- H. T. Ngo, X. Liu and K. A. Jolliffe, Chem. Soc. Rev., 2012, 41, 4928–4965 RSC.
- X. Tian, S. Hussain, C. de Pace, L. Ruiz-Pérez and G. Battaglia, Chem. – Asian J., 2019, 14, 509–526 CAS.
- F. Dumur, Synth. Met., 2014, 195, 241–251 CrossRef CAS.
- C. Bizzari, E. Spuling, D. M. Knoll, D. Volz and S. Bräse, Coord. Chem. Rev., 2018, 373, 49–82 CrossRef.
- S. Di Bella, A. Colombo, C. Dragonetti, S. Righetto and D. Roberto, Inorganics, 2018, 6, 133 CrossRef CAS.
- S. Di Bella, Dalton Trans., 2021, 50, 6050–6063 RSC.
- G. Consiglio, I. P. Oliveri, S. Failla and S. Di Bella, Molecules, 2019, 24, 2514 CrossRef CAS PubMed.
- C. T. L. Ma and M. J. MacLachlan, Angew. Chem., Int. Ed., 2005, 44, 4178–4182 CrossRef CAS PubMed.
- A. W. Kleij, Chem. – Eur. J., 2008, 14, 10520–10529 CrossRef CAS PubMed.
- A. W. Kleij, Dalton Trans., 2009, 4635–4639 RSC.
- L. Leoni and A. Dalla Cort, Inorganics, 2018, 6, 42 CrossRef.
- I. P. Oliveri, G. Maccarrone and S. Di Bella, J. Org. Chem., 2011, 76, 8879–8884 CrossRef CAS PubMed.
- G. Forte, I. P. Oliveri, G. Consiglio, S. Failla and S. Di Bella, Dalton Trans., 2017, 46, 4571–4581 RSC.
- J. Cheng, X. Ma, Y. Zhang, J. Liu, X. Zhou and H. Xiang, Inorg. Chem., 2014, 53, 3210–3219 CrossRef CAS PubMed.
- E. C. Escudero-Adán, J. Benet-Buchholz and A. W. Kleij, Inorg. Chem., 2007, 46, 7265–7267 CrossRef PubMed.
- L. San Felices, E. C. Escudero-Adán, J. Benet-Buchholz and A. W. Kleij, Inorg. Chem., 2009, 48, 846–853 CrossRef CAS PubMed.
- G. Salassa and L. Salassa, ACS Omega, 2021, 6, 7240–7247 CrossRef CAS PubMed.
- M. E. Carnes, M. S. Collins and D. W. Johnson, Chem. Soc. Rev., 2014, 43, 1825–1834 RSC.
- M. Lalonde, W. Bury, O. Karagiaridi, Z. Brown, J. T. Hupp and O. K. Farha, J. Mater. Chem. A, 2013, 1, 5453–5468 RSC.
- P. A. Vigato, S. Tamburini and L. Bertolo, Coord. Chem. Rev., 2007, 251, 1311–1492 CrossRef CAS.
- Y. Xia, D. Qiu and J. Wang, Chem. Rev., 2017, 117, 13810–13889 CrossRef CAS PubMed.
- J. Cheng, X. Zhou and H. Xiang, Analyst, 2015, 140, 7082–7115 RSC.
- G. Consiglio, S. Failla, P. Finocchiaro, I. P. Oliveri, R. Purrello and S. Di Bella, Inorg. Chem., 2010, 49, 5134–5142 CrossRef CAS PubMed.
- I. P. Oliveri, S. Failla, G. Malandrino and S. Di Bella, New J. Chem., 2011, 35, 2826–2831 RSC.
- I. P. Oliveri and S. Di Bella, Tetrahedron, 2011, 67, 9446–9449 CrossRef CAS.
- S. Mirabella, I. P. Oliveri, F. Ruffino, G. Maccarrone and S. Di Bella, Appl. Phys. Lett., 2016, 109, 143108 CrossRef.
- G. Consiglio, I. P. Oliveri, C. Cacciola, G. Maccarrone, S. Failla and S. Di Bella, Dalton Trans., 2020, 49, 5121–5133 RSC.
- M. Strianese, D. Guarnieri, M. Lamberti, A. Landi, A. Peluso and C. Pellecchia, Inorg. Chem., 2020, 59, 15977–15986 CrossRef CAS PubMed.
- G. Munzi, S. Failla and S. Di Bella, Analyst, 2021, 146, 2144–2151 RSC.
- G. Munzi, G. Consiglio, S. Failla and S. Di Bella, Inorganics, 2021, 9, 49 CrossRef CAS.
- I. P. Oliveri, G. Forte, G. Consiglio, S. Failla and S. Di Bella, Inorg. Chem., 2017, 56, 14206–14213 CrossRef CAS PubMed.
- G. Consiglio, I. P. Oliveri, S. Failla and S. Di Bella, Inorg. Chem., 2016, 55, 10320–10328 CrossRef CAS PubMed.
- I. Bertini and C. Luchinat, Coord. Chem. Rev., 1996, 150, 77–110 CrossRef.
- K. Miyamura, K. Satoh and Y. Hohshi, Bull. Chem. Soc. Jpn., 1989, 62, 45–50 CrossRef CAS.
- W. C. Chan, H. M. Saad, K. S. Sim, V. S. Lee, C. W. Ang, K. Y. Yeong and K. W. Tan, Spectrochim. Acta, Part A, 2021, 262, 120099 CrossRef CAS PubMed.
- A. Paul, L. M. D. R. S. Martins, A. Karmakar, M. L. Kuznetsov, M. F. C. Guedes da Silva and A. J. L. Pombeiro, Molecules, 2020, 25, 2644 CrossRef CAS PubMed.
- A. E. Stacy, D. Palanimuthu, P. V. Bernhardt, D. S. Kalinowski, P. J. Jansson and D. R. Richardson, J. Med. Chem., 2016, 59, 4965–4984 CrossRef CAS PubMed.
- D. De, S. Neogi, E. C. Saňudo and P. K. Bharadwaj, Chem. – Eur. J., 2015, 21, 17422–17429 CrossRef CAS PubMed.
- C. M. Flynn Jr., Chem. Rev., 1984, 84, 31–41 CrossRef.
- C. Brosset, Acta Chem. Scand., 1952, 6, 910–940 CrossRef CAS.
- A. R. Davis and D. E. Irish, Inorg. Chem., 1968, 7, 1699–1704 CrossRef CAS.
- E. C. Niederhoffer, J. H. Timmons and A. E. Martell, Chem. Rev., 1984, 84, 137–203 CrossRef CAS.
- T. Kurahashi and H. Fujii, Inorg. Chem., 2013, 52, 3908–3919 CrossRef CAS PubMed.
- D. H. Kim, U. S. Shin and C. E. Song, J. Mol. Catal. A: Chem., 2007, 271, 70–74 CrossRef CAS.
- E. Liu, Y. Z. Zhang, J. Tan, C. Yang, L. Li, J. A. Golen, A. L. Rheingold and G. Zhang, Polyhedron, 2015, 102, 41–47 CrossRef CAS.
- A. Huber, L. Müller, H. Elias, R. Klement and M. Valko, Eur. J. Inorg. Chem., 2005, 1459–1467 CrossRef CAS.
- A. A. Khandar, B. Shaabani, F. Belaj and A. Bakhtiari, Polyhedron, 2006, 25, 1893–1900 CrossRef CAS.
- Y.-L. Zhang, W.-J. Ruan, X.-J. Zhao, H.-G. Wang and Z.-A. Zhu, Polyhedron, 2003, 22, 1535–1545 CrossRef CAS.
- E. E. Hardy, M. A. Eddy, B. A. Maynard and A. E. V. Gorden, Dalton Trans., 2016, 45, 14243–14251 RSC.
- H. Temel, S. İlhan, M. Şekerci and R. Ziyadanoğullari, Spectrosc. Lett., 2002, 35, 219–228 CrossRef CAS.
- Q. Wu, Qi Zi, J. Li and Y. Li, Z. Kristallogr. – New Cryst. Struct., 2019, 234, 269–270 CrossRef CAS.
- H. Köksal, M. Dolaz, M. Tümer and S. Serin, Synth. React. Inorg. Met.-Org. Chem., 2001, 31, 1141–1162 CrossRef.
- I. P. Oliveri and S. Di Bella, Dalton Trans., 2017, 46, 11608–11614 RSC.
- M. Cano, L. Rodríguez, J. C. Lima, F. Pina, A. Dalla Cort, C. Pasquini and L. Schiaffino, Inorg. Chem., 2009, 48, 6229–6235 CrossRef CAS PubMed.
- S. J. Wezenberg, E. C. Escudero-Adán, J. Benet-Buchholz and A. W. Kleij, Chem. – Eur. J., 2009, 15, 5695–5700 CrossRef CAS PubMed.
- M. V. Escárcega-Bobadilla, M. Martínez-Belmonte, E. Martin, E. C. Escudero-Adán and A. W. Kleij, Chem. – Eur. J., 2013, 19, 2641–2648 CrossRef PubMed.
- I. Persson, K. C. Dash and Y. Kinjo, Acta Chem. Scand., 1990, 44, 433–442 CrossRef CAS.
- D. Feakins, A. S. Willmott and A. R. Willmott, J. Chem. Soc., Faraday Trans. 1, 1973, 69, 122–131 RSC.
- X. Liu, C. Manzur, N. Novo, S. Celedón, D. Carrillo and J.-R. Hamon, Coord. Chem. Rev., 2018, 357, 144–172 CrossRef CAS.
- G. Ahumada, J. Oyarce, T. Roisnel, S. Kahlal, M. A. del Valle, D. Carrillo, J.-Y. Saillard, J.-R. Hamon and C. Manzur, New J. Chem., 2018, 42, 19294–19304 RSC.
- H. Kargar, M. Ashfaq, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, K. S. Munawar and M. N. Tahi, Inorg. Chim. Acta, 2022, 536, 120878 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional 1H NMR, UV/vis spectroscopy, and mass spectrometric data. See DOI: https://doi.org/10.1039/d2dt01448c |
| This journal is © The Royal Society of Chemistry 2022 |