Synthesis and the spectral, electrochemical, and nonlinear optical properties of β-dicyanovinyl-appended ‘push–pull’ porphyrins†
Abstract
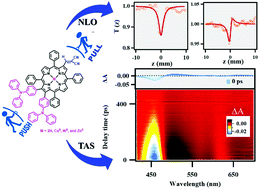
A series of “push–pull” porphyrins, MTPP(MN)(TPA)2 (M = 2H, CuII, NiII, and ZnII), having triphenylamine (TPA) and dicyanovinyl (DCN) groups at antipodal positions were synthesized and characterised by UV-Vis, fluorescence and NMR spectroscopic techniques, MALDI-TOF mass spectrometry, cyclic voltammetry, DFT, and elemental analysis, which were then further utilized for third-order nonlinear optical measurements under mild conditions using femtosecond laser pulses. Remarkably, MTPP(MN)(TPA)2 (M = 2H, CuII, NiII, and ZnII) exhibited 21–48 nm and 38–80 nm bathochromic shifts in B and Qx(0,0) bands as compared to the corresponding MTPPs (M = 2H, CuII, NiII, and ZnII); the results are consistent with the effect of enhanced resonance due to TPA and –I effect of DCN moieties. In cyclic voltammetry, the push–pull porphyrins exhibited a cathodic shift (0.13–0.51 V) in their first oxidation potential as compared to the precursor owing to the presence of electron-donating TPA groups. The third-order nonlinear optical responses were recorded using a single-beam femtosecond Z-scan technique to retrieve information about the nonlinear absorption and nonlinear refraction of the samples. The two-photon absorption coefficients (β) are in the range of 0.87 × 10−13 to 4.28 × 10−13 m W−1 and the nonlinear refractive index (n2) in the range of 1.21 × 10−19 to 7.36 × 10−19 m2 W−1. The ultrafast absorption dynamics of the ground-state bleaching (GSB) and photo-induced absorption (PIA) are monitored by femtosecond broadband transient absorption studies. The strong nonlinearity of these push–pull porphyrins makes them potential candidates for nonlinear optical and photonic device applications.


 Please wait while we load your content...
Please wait while we load your content...