Open Access Article
Open Access ArticleWater soluble organometallic small molecules as promising antibacterial agents: synthesis, physical–chemical properties and biological evaluation to tackle bacterial infections†
Ines
Bennour
a,
M. Núria
Ramos
b,
Miquel
Nuez-Martínez
a,
Jewel Ann Maria
Xavier
 a,
Ana B.
Buades
a,
Reijo
Sillanpää
c,
Francesc
Teixidor
a,
Ana B.
Buades
a,
Reijo
Sillanpää
c,
Francesc
Teixidor
 a,
Duane
Choquesillo-Lazarte
a,
Duane
Choquesillo-Lazarte
 d,
Isabel
Romero
d,
Isabel
Romero
 e,
Margarita
Martinez-Medina
d and
Clara
Viñas
e,
Margarita
Martinez-Medina
d and
Clara
Viñas
 *a
*a
aInstitut de Ciència de Materials de Barcelona, Consejo Superior de Investigaciones Científicas, Campus Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain. E-mail: clara@icmab.es; Fax: +34-935805729
bMicrobiology of Intestinal Diseases, Biology Department, Universitat de Girona, 17003 Girona, Spain
cDept. of Chemistry, University of Jyväskylä. FIN-40014, Jyvaskyla, Finland
dLaboratorio de Estudios Cristalográficos, IACT, CSIC-Universidad de Granada, Armilla, 18100 Granada, Spain
eDepartament de Química and Serveis Tècnics de Recerca, Universitat de Girona, C/M. Aurèlia Campmany, 69, E-17003 Girona, Spain
First published on 6th April 2022
Abstract
The Na[3,3′-Fe(8-I-1,2-C2B9H10)2] and Na[2,2′-M(1,7-C2B9H11)] (M = Co3+, Fe3+) small molecules are synthesized and the X-ray structures of [(H3O)(H2O)5][2,2′-Co(1,7-C2B9H11)2] and [Cs(MeCN)][8,8′-I2-Fe(1,2 C2B9H10)2], both displaying a transoid conformation of the [M(C2B9)2]− framework, are reported. Importantly, the supramolecular structure of [(H3O)(H2O)5][2,2′-Co(1,7-C2B9H11)2] presents 2D layers leading to a lamellar arrangement of the anions while the cation layers form polymeric water rings made of six- and four-membered rings of water molecules connected via OH⋯H hydrogen bonds; B–H⋯O contacts connect the cationic and anionic layers. Herein, we highlight the influence of the ligand isomers (ortho-/meta-), the metal effect (Co3+/Fe3+) on the same isomer, as well as the influence of the presence of the iodine atoms on the physical–chemical and biological properties of these molecules as antimicrobial agents to tackle antibiotic-resistant bacteria, which were tested with four Gram-positive bacteria, five Gram-negative bacteria, and three Candida albicans strains that have been responsible for human infections. We have demonstrated an antimicrobial effect against Candida species (MIC of 2 and 3 nM for Na[3,3′-Co(8-I-1,2-C2B9H10)2] and Na[2,2′-Co(1,7-C2B9H11)2], respectively), and against Gram-positive and Gram-negative bacteria, including multiresistant MRSA strains (MIC of 6 nM for Na[3,3′-Co(8-I-1,2-C2B9H10)2]). The selectivity index for antimicrobial activity of Na[3,3′-Co(1,2-C2B9H11)2] and Na[3,3′-Co(8-I-1,2-C2B9H10)2] compounds is very high (165 and 1180, respectively), which reveals that these small anionic metallacarborane molecules may be useful to tackle antibiotic-resistant bacteria. Moreover, we have demonstrated that the outer membrane of Gram-negative bacteria constitutes an impermeable barrier for the majority of these compounds. Nonetheless, the addition of two iodine groups in the structure of the parent Na[3,3′-Co(1,2-C2B9H11)2] had an improved effect (3–7 times) against Gram-negative bacteria. Possibly the changes in their physical–chemical properties make the meta-isomers and the ortho-di-iodinated small molecules more permeable for crossing this barrier. It should be emphasized that the most active metallabis(dicarbollide) small molecules are both transoid conformers in contrast to the ortho- [3,3′-Co(1,2-C2B9H11)2]− that is cisoid. The fact that these small molecules cross the mammalian membrane and have antimicrobial properties but low toxicity for mammalian cells (high selectivity index, SI) represents a promising tool to treat infectious intracellular bacteria. Since there is an urgent need for antibiotic discovery and development, this study represents a relevant advance in the field.
Introduction
Over the years, the rapid development and spread of new antimicrobial resistance (AMR) has forced the World Health Organization (WHO) to recognize it is a major risk to public health.1 AMR has made many antimicrobials ineffective in treating clinically ill patients. Even medical procedures such as major surgery, organ transplantation, and cancer chemotherapy have become very risky in the absence of effective antimicrobials for the prevention and treatment of nosocomial infections. It is estimated that nosocomial infections affect around 4.1 million people in the European Union each year, being around the 7%–8% of patients receiving healthcare in Spain.2 The leading cause of nosocomial infections throughout the world is the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species), most of them being multidrug-resistant isolates.3 Because of the drastic rise in antibiotic resistance, greater than the discovery of new antibiotics, there is an urgent need for the development and evaluation of novel effective antimicrobial agents active against drug-resistant forms.Nanomaterials, owing to their high specific surface area and abundant modification sites, have been shown to be promising weapons for cancer therapy4 and to possess a great potential as new antimicrobial agents effective against resistant bacteria.5 Recent reviews update the advances in nanoparticles6 and metal complexes7 as emerging antibacterial nanomedicine for enhanced antibiotic therapy.
Most of the marketed small molecules8 are organic molecules, which incorporate nitrogen, oxygen, and halogens besides carbon and hydrogen, all of them the right-hand neighbors of carbon. Boron is an element which, despite being located on the left side of carbon in the Periodic Table, has the property to build molecules by covalent self-bonding.9 The neutral twelve-vertex icosahedral closo-carboranes C2B10H12, ortho-, meta-, or para-isomers, the anionic nido-carboranes and the anionic metallabis(dicarbollides) [M(C2B9H11)2]− (M = Co3+, Fe3+) (Fig. 1) rank among the most chemically and biologically stable small molecular compounds known.10–12
The most studied of the metallabis(dicarbollides) is [3,3′-Co(1,2-C2B9H11)2]− anion, [1]−. Prominent features of the [1]− anion are 3D aromaticity,10,13 thermal, chemical (withstanding strong acid, moderate base, high temperatures and intense radiation)14 and biological (neither degradation nor chemical modification compounds were identify after cell uptake) stability,15,16 and solubility in both polar and nonpolar solvents.11 Besides these, other characteristic properties of this anion are: (i) the negative charge of [1]− is spread all over the molecule,10,17 (ii) [1]− aggregates in cisoid conformation in aqueous solution by means of B–H⋯H–Ccluster dihydrogen bond formation,18 and (iii) although lacking the classical hydrophilic–hydrophobic structure,19 [1]− shows amphiphilic behavior,20 which was experimentally proved by a study based on the electrolysis of water.21 Furthermore, the anion [1]−, the 3D shape of which is reminiscent of the Greek letter θ and has a size of 1.1 nm × 0.6 nm, produces strong dihydrogen-bond B–H⋯H–N interactions with the amine groups of amino acids22 and proteins.23 Moreover, [1]−, which can cross through synthetic lipid membranes without disrupting membrane integrity,24 displays low toxicity in vitro15,16 and in vivo16,25 and high uptake by relevant cancer cells,15,26 accumulating in vitro within the nuclei of living cells.16 Additionally, the anionic [M(C2B9H11)2]− clusters can be modified at the different vertexes by halogenation,27–29 and are responsible for additional physicochemical properties.10,29,30 Recently, amphiphilic inorganic anionic [1]− clusters have been proposed to stabilize oil-in-water nano-emulsions of poorly water soluble drugs.31 The increasing evidence that boron cluster compounds are promising antimicrobial agents has been reviewed recently.32
There are two groups of oral antibacterial agent on the market, all of them small organic molecules: one comprises molecules in the molecular weight range of 340–450 Da and the other molecules of 700–900 Da.33 Following our studies on metallacarboranes in medicinal chemistry,34 we report herein high-yield syntheses of the sodium salts of four isomeric pristine metallabis(dicarbollide) molecules (321–324 Da) and their di-iodinated derivatives (572–576 Da) as abiotic, small, purely inorganic molecules for antimicrobial therapy. Chart 1 displays the general schematic representation of all the small inorganic anionic [M(C2B9H11−nXn)2]−, (M = Co3+, Fe3+, n = 1, X = H or I), molecules studied in this paper as antimicrobial agents.
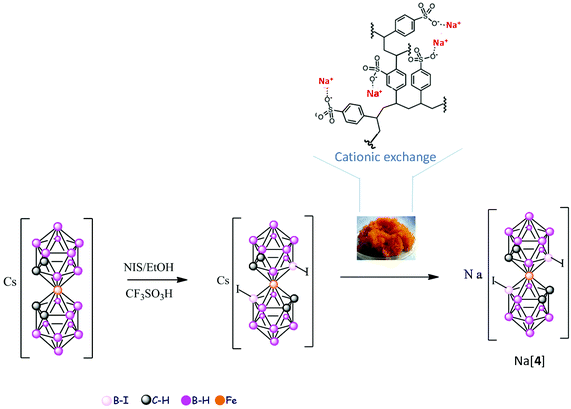
ortho-Isomers are: Na[3,3′-M(1,2-C2B9H11)2], M = Co, Na[1]; Fe, Na[2]. Na[3,3′-M(8-I-1,2-C2B9H10)2], M = Co, Na[3]; Fe, Na[4]. meta-Isomers are: Na[2,2′-Co(1,7-C2B9H11)2], M = Co, Na[5]; Fe, Na[6].
Herein, we highlight the effect of the ligand isomers (ortho-/meta-), the metal effect (Co3+/Fe3+) on the same isomer as well as the influence of the presence of iodine atoms on the physical and chemical properties of these small inorganic anionic molecules with activity against four Gram-positive bacteria, five Gram-negative bacteria, and three Candida albicans strains that have been responsible for human infections. A structure–activity relationship has been proposed, which clearly supports the antimicrobial activity of these pristine metallabis(dicarbollide) complexes.
Results and discussion
The synthesis of the small anionic sandwich metallabis(dicarbollides) is achieved by a metal complexation reaction of the nido-[C2B9H11]2− ligands in several conditions (see scheme S1–S4 in the ESI†).35 There are two positional isomers of the nido [C2B9H11]2− carboranyl ligands (Fig. 2a): the isomer ortho-([7,8-C2B9H11]2−) in which the two carbon atoms are connected and, the isomer meta-([7,9-C2B9H11]2−) wherein a boron atom is located in between the two carbon atoms in the η5C2B3 coordinating face. Depending on the starting [C2B9H11]2−nido carboranyl ligand, two positional ortho- and meta-metallabis(dicarbollide) isomers are obtained, whose empirical formulae are [3,3′-M(1,2-C2B9H11)2]− and [2,2′-M(1,7-C2B9H11)2]−, (M = Co3+, Fe3+), respectively (Fig. 2). Fig. 1 displays the numbering of these two platforms.The four anionic ortho- and meta-metallabis(dicarbollide) isomers are abbreviated as [1]−, [2]−, [5]− and [6]− for M = Co3+ and Fe3+, respectively. As shown in Fig. 2, five different conformations that provide a different dipole moment36 can be found for the ortho-metallabis(dicarbollide) isomer: cisoid-1, gauche-1, transoid, gauche-2 and cisoid-2 (being cisoid-1 and cisoid-2 as well as gauche-1 and gauche-2 equivalent), while three different conformations are found for the meta-metallabis(dicarbollide) isomer.
The sodium salts of [1]−, [2]− and [3]− were synthesized as previously reported by us (see Schemes S1–S3 in the ESI†).18,37 The new Na[5] and Na[6] species have been obtained in this article by means of a cationic exchange resin of the corresponding tetramethyl ammonium or cesium salts and are fully characterized by spectroscopic techniques IR, 1H, 1H{11B}, 11B and 11B{1H} NMR, MALDI-TOF-MS, UV-vis, cyclic voltammetry (CV) as well as by thermal analysis (TGA/DSC) as stated at the Experimental section and ESI.†
Despite multiple attempts, no good crystals of Na[5] suitable for X-ray diffraction studies, to compare with the reported Na[1], could be obtained,38 but suitable crystals of the protonated salt of [5]− were obtained from aqueous solution (Fig. 3).
The Cs[3,3′-Fe(8-I-1,2-C2B9H10)2], Cs[4], was synthesized in this article from [2]− and N-iodosuccinamide by a modification of the original synthesis by Kazheva et al.39 This procedure permits the isolation of Cs[4] with an easy purification step. Crystals of Cs[4] were grown from an acetonitrile solution (Fig. 4). The corresponding water soluble small molecule Na[4] was obtained from the Cs[4] salt by means of cationic exchange resin (Scheme 1).
Crystal structures of H[5] and Cs[4]
An examination of the Cambridge Structural Database40 showed one hit for the diamagnetic Co3+ unsubstituted parent [5]−,35e and one for the paramagnetic [4]− framework.39 The CDS refcodes are HODGAL (transoid rotamer),35e and GANQIY (transoid rotamer),39 respectively. It is to be noted that none of the reported crystal structures of the ortho- and meta-parent metallabis(dicarbollide) frameworks contain a proton as counter ion. The volume (286.5 Å3) and molecular shape (cylindrical with one of the moments larger than the other two) of the [2]− anion was determined by Mingos et al. using the VOLUME program.41The acetonitrile molecule forms a bridge between Cs+ ions, which form linear chains (Fig. 5) along the c axis (Cs–N distances 3.137(5) Å; Cs⋯Cs distances 4.46005(15) Å). The Cs cation has a coordination number of 8: two I, two N, two H from B9 with Cs–H distances of 3.147 Å, and two H from B12 with Cs–H distances of 3.280 Å. The linear Cs chains results in a body center type structure in which the Cs⋯Cs chain distances are 12.514 and 12.642 Å, thus generating a supramolecular structure showing alternating layers of iron complex anions and Cs+ cations plus acetonitrile solvent molecules stacked along the a axis.
Both related cobalt and iron complexes Cs[3] and Cs[4] exhibit similar packing indexes (0.726 and 0.725, respectively). Nonetheless, the distances observed for Cs⋯Cs and Cs⋯I interactions are shorter in [Cs(MeCN)][4] than in the analogue Cs[3] (Cs⋯Cs distance 6.813 Å and Cs⋯I distance 4.140 Å), which can be attributed to the acetonitrile solvent in the crystal packing.
Physico-chemical properties of metallabis(dicarbollides) and their di-iodinated derivatives
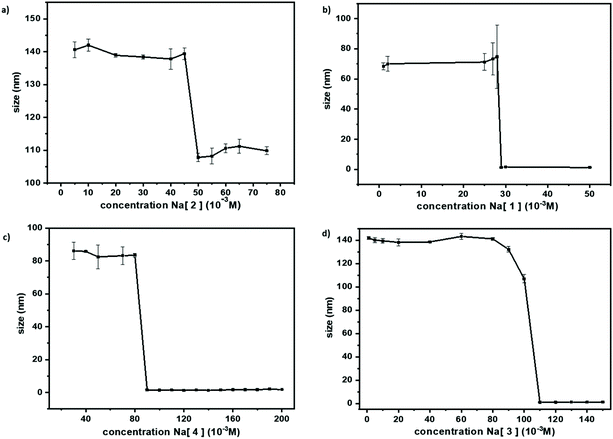
As mentioned in the introduction, there are several published studies on the properties of [1]− but, to the best of our knowledge, studies pertaining to [2]−, [5]− and [6]− are scarce. Herein, we report on the physico-chemical, spectroscopic, and electrochemical studies of these less-known small inorganic molecules. The different behavior of metallabis(dicarbollide) isomers (ortho/meta) in aqueous solution have been studied by dynamic light scattering (DLS), and transmission electron microscopy (TEM and CryoTEM).In medicinal chemistry, it is crucial to know the compounds’ behavior in aqueous solution. Hence, we performed dynamic light scattering of the less studied metallabis(dicarbollides), Na[2], Na[4], Na[5], and Na[6] in aqueous solutions at different concentrations to obtain information on their possible self-assembly. Fig. 6 displays the DLS measurements in aqueous solution of the reported small ferrabis(dicarbollide) anionic molecules as well as the previously reported Na[1]18 for comparison. A decrease in the hydrodynamic diameter of the aggregates was observed when the concentration increased. At a concentration of 50 mM, a change in aggregate size was observed for Na[2] from 140 nm to 108 nm. For the iodinated derivative, Na[4], the change in size was observed at 90 mM and was from 83.5 nm to 1.5 nm. In all four ortho-metallabis(dicarbollide) isomers (Fig. 6), a decrease in the hydrodynamic diameter of the aggregates was observed by DLS experiments when the concentration was increased, which could be attributed to a Coulomb explosion of the closely packed monolayer aggregates into small micelles. The aggregate size of Na[3] at 110 mM changes from 1.11 nm to 110 nm at 107 mM.44
In order to visualize the large and small aggregates of ferrabis(dicarbollides) TEM images were taken. In the case of the parent Na[2], CryoTEM and TEM images of 1 mM aqueous solution were obtained for comparison (Fig. 7).
 | ||
| Fig. 7 CryoTEM and TEM images of an aqueous solution 1 mM of Na[2]. Bar length is 100 nm in the CryoTEM and 50 nm in the TEM. | ||
In the case of the meta isomers, Na[5] and Na[6], no aggregates were observed by DLS measurements in the concentration range 120 – 1 mM and 110 – 1 mM respectively, which is in agreement with the dynamic 1H{11B} NMR study (ESI†). To summarize, the Na[1] and Na[5] isomers behave differently in aqueous solution: the ortho-isomer forms aggregates while the latter do not.
Table 1 summarizes the redox potential Ered, ΔE values (ΔE = Ered − Eox) and E1/2 (E1/2 = (Ered + Eox)/2) of all the redox couples related to Fc+/Fc. E1/2 is the half wave potential associated with the reversible redox Co3+/Co2+ process. We observed that the E1/2 potential of the studied anionic charged metallabis(dicarbollide) complexes can be tuned by changing the metal, changing the positional isomer of the ligands or by introducing an iodo substituent on the pentagonal coordinating η5-C2B3 face of the ligands. By changing the metal, the E1/2 potential decreases +1.1 V from [1]− to [2]− (−1.81 V to −0.73 V, respectively) and the same tendency is observed in the two redox couples [3]− to [4]− (−1.33 V to −0.36 V, respectively).
| Metal | Complex | E 1/2 (V) | ΔE (V) | E red (V) |
|---|---|---|---|---|
| Co3+/Co2+ | Na[1] | −1.81 (ref. 46) | 0.10 | −1.86 |
| Na[5] | −1.55 | 0.12 | −1.60 | |
| Na[3] | −1.33 (ref. 46) | 0.19 | −1.42 | |
| Co4+/Co3+ | Na[1]− | 1.16 | 0.09 | 1.12 |
| Na[5] | 1.44 | 0.10 | 1.38 | |
| Na[3] | 1.07 | 0.23 | 0.95 | |
| Fe3+/Fe2+ | Na[2] | −0.73 | 0.10 | −0.78 |
| Na[6] | −0.79 | 0.11 | −0.85 | |
| Na[4] | −0.36 | 0.10 | −0.41 | |
| Fe4+/Fe3+ | Na[2] | 0.75 | 0.16 | 0.67 |
| Na[6] | 0.66 | 0.10 | 0.61 | |
| Na[4] | 1.11 | 0.14 | 1.04 | |
A small variation in the E1/2 potential is observed between the isomers [1]− and [5]− (−1.81 V to 1.55 V, respectively) as well as when comparing [2]− with [6]− (−0.73 V to −0.79 V, respectively), while in [3]− and [4]−, the presence of two iodo groups at the B(8,8′) positions at the η5-C2B3 face of either [1]− or [2]− decreases the E1/2 potential +0.48 V and +0.37 V, respectively.
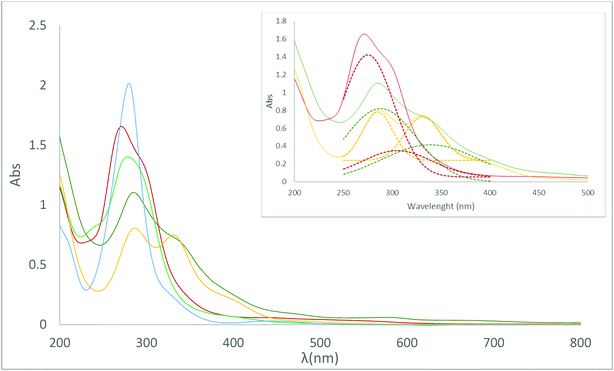
Table 2 displays the UV-vis spectra of the studied [M(C2B9H11)2]− complexes in water. λ positions and ε values are reported and were calculated following line fitting analysis.
| Complex | Max λ (0.08 mM) | Abs (0.08 mM) | ε (L mmol−1 cm−1) |
|---|---|---|---|
| Na[4] | 333.5 | 0.720 | 9.000 |
| 285.5 | 1.104 | 13.80 | |
| Na[2] | 302.5 | 1.217 | 15.21 |
| 271 | 1.665 | 20.81 | |
| Na[6] | 278.5 | 1.401 | 17.51 |
| Na[3] | 331 | 0.747 | 9.338 |
| 286.5 | 0.804 | 10.05 | |
| Na[1] | 280 | 2.013 | 25.16 |
| Na[5] | 280 | 2.247 | 28.08 |
The compounds exhibit intense bands around 270–280 nm, and in the case of Na[3] a weak band is also observed at 331 nm. These intense bands have been assigned to charge transfer from the ligand framework towards the metal centre (LMCT).47
The presence of cobalt in the metallacarborane produces a bathochromic shift of the absorptions versus iron, and lower wavelengths have been observed for the Fe compounds in comparison with the Co ones. The UV-vis spectra do not seem to be affected by the different isomers ortho- and meta- in the case of cobaltabis(dicarbollide) compounds Na[1] and Na[5] (λ = 280 nm). However, an increase in wavelength takes place for the meta-isomer in the case of Na[6] (λ = 278.5 nm) in comparison with the ortho-isomer Na[2] (λ = 271 nm). For the iodo-derivatives, Na[3] and Na[4], a shift to the visible spectrum, was observed (λ = 286.5 and λ = 285.5 nm) with respect to the corresponding unsubstituted metallacarboranes Na[o-COSAN] and Na[o-FESAN] (λ = 280 and λ = 271 nm), respectively. A similar behavior has been observed for the dichloro-derivatives, although in the latter the bathochromic shift, caused by the chlorine substituents, is greater.28b
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S), which is the concentration (mol L−1) of its saturated aqueous solution, is an important factor that affects its bioavailability.48 There are several factors affecting the solubility of a compound such as the size and shape of the molecule, its polarity and hydrophobicity, as well as its ability to participate in intra- and intermolecular hydrogen or dihydrogen bonding. Taking advantage of the fact that meta-metallabis(dicarbollide) small molecules absorb at the UV-vis region, we used a quantitative method based on UV-vis spectroscopy to calculate the log
S), which is the concentration (mol L−1) of its saturated aqueous solution, is an important factor that affects its bioavailability.48 There are several factors affecting the solubility of a compound such as the size and shape of the molecule, its polarity and hydrophobicity, as well as its ability to participate in intra- and intermolecular hydrogen or dihydrogen bonding. Taking advantage of the fact that meta-metallabis(dicarbollide) small molecules absorb at the UV-vis region, we used a quantitative method based on UV-vis spectroscopy to calculate the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S of the two meta- metallabis(dicarbollide) small molecules, whose values are shown in Table 3. In this method, the UV-vis absorption of the aqueous solution containing the metallabis(dicarbollide) was measured to study its molar absorptivity (εmax); as this is proportional to the concentration of the corresponding metallabis(dicarbollide), we obtained the aqueous solubility value of each complex. Hence, UV-vis spectroscopy has been a good technique for estimating the aqueous solubility, and the log
S of the two meta- metallabis(dicarbollide) small molecules, whose values are shown in Table 3. In this method, the UV-vis absorption of the aqueous solution containing the metallabis(dicarbollide) was measured to study its molar absorptivity (εmax); as this is proportional to the concentration of the corresponding metallabis(dicarbollide), we obtained the aqueous solubility value of each complex. Hence, UV-vis spectroscopy has been a good technique for estimating the aqueous solubility, and the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S values are 3.24 for Na[5] and 3.15 for Na[6]. The meta-metallabis(dicarbollide) isomers, which do not aggregate, display the highest aqueous solubility, while the 8,8′-I2-o-metallabis(dicarbollide) isomers display the lowest (see Table 3).
S values are 3.24 for Na[5] and 3.15 for Na[6]. The meta-metallabis(dicarbollide) isomers, which do not aggregate, display the highest aqueous solubility, while the 8,8′-I2-o-metallabis(dicarbollide) isomers display the lowest (see Table 3).
| Parameters | [1]− | [2]− | [3]− | [4]− | [5]− | [6]− |
|---|---|---|---|---|---|---|
| Size (nm) = L × W(max) L = length and W = width (ref. 50) | 1.04 × 0.53 (ref. 38) | 1.01 × 0.56 (ref. 49) | 1.02 × 0.75 (ref. 27a) | 1.02 × 0.75 | 1.01 × 0.54 | 1.01 × 0.54 (ref. 60) |
| Molecular weight of the anion | 323.75 | 320.66 | 575.54 | 572.45 | 323.75 | 320.66 |
| Rotamer | Cisoid | Cisoid | Transoid | Transoid | Transoid | Transoid |
| Intramolecular interactions | No | No | Cc–H⋯I | Cc–H⋯I | No | No |
| Intermolecular interactions in aqueous solution | Yes | Yes | Yes | Yes | No | No |
| Aggregate formation in H2O | Yes (ref. 18 and 20c) | Yes | Yes (ref. 18) | Yes | No | No |
| DLS | Aggregates (ref. 44) (d = 64 nm) in the range 1 < c < 29 mM | Aggregates (d = 140 nm) in the range 1 < c < 50 mM | Aggregates (ref. 44) (d = 107 nm) in the range 1 < c < 110 mM | Aggregates (d = 83.5 nm) in the range 1 < c < 80 mM | — | — |
| Aggregates (d = 1.4 nm) c > 29 mM | Aggregates (d = 108 nm) c > 50 mM | Aggregates (d = 1.1 nm) at c > 110 mM | Aggregates (d = 1.5 nm) at c > 80 mM | |||
| Solubility in H2O (mM) | 1509 (ref. 26) | 1247 | 210 (ref. 26b) | 374 | 1726 | 1.400 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S S |
3.18 | 3.10 | 2.32 | 2.57 | 3.24 | 3.15 |
| Lipophilicity (P) | 43.7 | 45.7 | 151.0 | 99.3 | 26.0 | 35.2 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
1.64 | 1.66 | 2.18 | 2.00 | 1.41 | 1.55 |
| E 1/2 M3+/2+(in V reference Fc+/Fc) | −1.80 | −0.73 | −1.33 | −0.36 | −1.55 | −0.79 |
Besides aqueous solubility, a drug's lipophilicity is a key physicochemical parameter because it determines its solubility, its permeability through membranes, and therefore its absorption, biodistribution and clearance. Lipophilicity is expressed by the n-octanol/water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P).51 The as-found P values are 43.7 (log
P).51 The as-found P values are 43.7 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1.64) for Na[1],44 45.7 (log
P = 1.64) for Na[1],44 45.7 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1.66) for Na[2], 151.0 (log
P = 1.66) for Na[2], 151.0 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 2.18) for Na[3],44 99.3 (log
P = 2.18) for Na[3],44 99.3 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 2.00) for Na[4], 26.0 (log
P = 2.00) for Na[4], 26.0 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1.41) for Na[5] and 35.2 (log
P = 1.41) for Na[5] and 35.2 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1.55) for Na[6].
P = 1.55) for Na[6].
When comparing the two isomers of the same metal, Na[1]/Na[5] and Na[2]/Na[6], the ortho-isomers are more lipophilic than the corresponding meta-isomers (approximately 1.7 and 1.3-fold for the Co and Fe complexes, respectively). While comparing the same isomer but a different metal, (Na[1]/Na[2] and Na[5]/Na[6], the iron provides a more lipophilic character than the cobalt, with the effect being higher in the meta-isomers. When comparing the effect of the two iodine atoms, Na[1]/Na[3] and Na[2]/Na[6], the lipophilicity increased with the presence of the two iodine atoms in both the iodinated couples (approximately 3.5 and 2.2-fold for the Co and Fe complexes, respectively). Consequently, the ligand's isomerism (ortho-/meta-) as well as the nature of the metal (Co/Fe) significantly affects the lipophilicity of the small metallabis(dicarbollide) molecule.
Lipophilicity is a major factor influencing passive transfer to or from the brain across the blood–brain barrier (BBB). Typically, for a radiotracer to be considered as a potential efficient molecular imaging probe in the living human brain with positron emission tomography (PET), its partition coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) should range between 2.0–3.5.52 The metallabis(dicarbollide) complexes with log
P) should range between 2.0–3.5.52 The metallabis(dicarbollide) complexes with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P in this range are the di-iodinated Na[3] and Na[4] (Table 3).
P in this range are the di-iodinated Na[3] and Na[4] (Table 3).
Antimicrobial studies
Although biological studies on cancer cells have been reported for [1]− and [5]−,26 no studies of the small inorganic amphiphilic molecule [M(C2B9H11)2]− (M = Co3+, Fe3+) parents as antimicrobial agents have been reported yet. Chart 2 displays the reported cobaltabis(dicarbollide) derivatives with tested antimicrobial properties; the three compounds with the amino group are zwitterionic while the others are mono- and di-anionic. | ||
| Chart 2 Reported cobaltabis(dicarbollides) with effective antimicrobial properties.53,54 | ||
Chart 1 shows the six small anionic metallabisdicarbollide molecules tested in this study to predict their potential application as antimicrobial agents. Herein, the influence of the [C2B9H11]2− ligand isomers (ortho-/meta-), the metal effect (Co3+/Fe3+) on the same isomer, as well as the influence of the presence of the iodine atoms on the structure–activity relationship of these as promising antimicrobial agents to tackle antibiotic-resistant bacteria was studied.
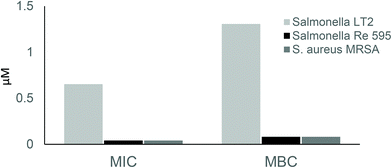
All the metallabis(dicarbollides) tested in the study had a better effect on yeast and Gram-positive bacteria than on Gram-negative bacteria. The minimum inhibitory concentration (MIC) values ranged from 0.002 to 0.082 μM for Candida species, from 0.006 to 0.041 μM for Gram-positives and from 0.050 to 0.653 μM for Gram-negatives (Table 4). The minimum bactericidal concentration (MBC) values indicated that these compounds had a bactericidal/fungicidal effect, since the ratio MBC/MIC was ≤2. However, while previously tested [1]− derivatives with a cisoid conformation were highly effective against Gram-positives,53a,54 the parent metallabis(dicarbollides) studied here had a reduced antimicrobial effect towards this group of bacteria, but increased potency against Gram-negative and Candida species.
| Strain | Na[1] | Na[3] | Na[5] | Na[2] | Na[4] | Na[6] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| a Multidrug-resistant strains. | ||||||||||||
| Gram-negative bacteria | ||||||||||||
| E. coli – LF82a | 0.653 | 1.307 | 0.050 | 0.050 | 0.082 | 0.082 | 0.649 | 0.649 | 0.250 | 0.400 | 0.165 | 0.329 |
| E. coli WT – 190940 | 0.653 | 0.653 | 0.050 | 0.050 | 0.082 | 0.163 | 0.649 | 0.649 | 0.250 | 0.250 | 0.165 | 0.165 |
| E. coli BLEE – 192348a | 0.653 | 0.653 | 0.050 | 0.050 | 0.082 | 0.163 | 0.325 | 0.325 | 0.250 | 0.250 | 0.165 | 0.165 |
| P. aeruginosa WT – 190089 | 0.653 | 0.653 | 0.050 | 0.099 | 0.163 | 0.327 | 0.649 | 0.649 | 0.250 | 0.250 | 0.325 | 0.325 |
| P. aeruginosa IMI-R – 187182a | 0.653 | 0.653 | 0.050 | 0.099 | 0.163 | 0.327 | 0.649 | 0.649 | 0.250 | 0.250 | 0.325 | 0.325 |
| Gram-positive bacteria | ||||||||||||
| E. faecalis WT – 190093 | 0.041 | 0.082 | 0.012 | 0.012 | 0.041 | 0.041 | 0.041 | 0.041 | 0.025 | 0.025 | 0.021 | 0.021 |
| E. faecalis WT – 194844 | 0.041 | 0.041 | 0.012 | 0.012 | 0.041 | 0.041 | 0.041 | 0.041 | 0.025 | 0.025 | 0.021 | 0.021 |
| S. aureus WT – 180895 | 0.041 | 0.082 | 0.006 | 0.012 | 0.041 | 0.041 | 0.041 | 0.082 | 0.012 | 0.012 | 0.021 | 0.021 |
| S. aureus MRSA – 182851a | 0.041 | 0.082 | 0.006 | 0.012 | 0.041 | 0.041 | 0.041 | 0.082 | 0.025 | 0.050 | 0.010 | 0.021 |
| Yeast | ||||||||||||
| C. albicans – 180228 | 0.082 | 0.163 | 0.002 | >0.003 | 0.003 | >0.010 | 0.041 | 0.082 | 0.012 | 0.025 | 0.005 | >0.010 |
| C. albicans – 181721 | 0.082 | 0.163 | 0.002 | >0.003 | 0.003 | >0.010 | 0.041 | 0.082 | 0.012 | 0.025 | 0.005 | >0.010 |
| C. albicans – 191026 | 0.082 | 0.163 | 0.002 | >0.003 | 0.003 | >0.010 | 0.041 | 0.082 | 0.012 | 0.025 | 0.005 | >0.010 |
It was experimentally demonstrated by small- and wide-angle X-ray and neutron scattering that Na[1] has a rich self-assembly behavior, being able to form monolayer vesicles and micelles in diluted aqueous solution.20c Besides, theoretical DFT calculations using implicit solvent found that the cisoid rotamer (which has a polar and an apolar region) is the most stable form of Na[1] small anionic molecules in water.19 As stated at the beginning of this section, the DLS and NMR studies of the meta-metallabis(dicarbollide) indicated that these isomers do not produce aggregates and also that the crystal structure of H[5] presents a transoid conformation of the [5]− small anionic molecule, which organizes in 2D layers leading to a lamellar arrangement of only [5]− units. The transoid rotamers present in the meta-metallabis(dicarbollides), which lacks a “hydrophilic head” and a “hydrophobic tail” compared with cisoid rotamers present in the ortho-metallabis(dicarbollides), may be the key factor for meta-isomers being more effective than the ortho-isomers against Gram-negative bacteria.
In order to discern if the translocation process of the metallabis(dicarbollide) small molecules is related to their conformation, cisoid for the ortho- and transoid for the meta-, the transoid conformation of the ortho-metallabis(dicarbollides) was forced with the introduction of an iodine atom in each B(8) vertex of each complex's ligand. The transoid rotamer is fixed in both di-iodinated [3]− and [4]− small molecules because of two factors: (i) the repulsion of the three lone pairs of electrons of the iodine atom of the B(8)–I bond in each ligand of the complexes and (ii) the formation of two Cc–H⋯I–B(8) intramolecular bonds between the Cc–H vertex from one ligand and the I–B(8) vertex from the other one. The crystal structures of Cs[3] and [4]− (codes refcodes DEXPIF42 and GANQIY27a), which present two-fold symmetry anions with the two iodine atoms in the trans position, support the most stable rotamer, i.e. transoid, in both di-iodinated metallabis(dicarbollides). Additionally, it was also proved that the [3]− small anionic molecule combines the properties of purely organic surfactants and purely inorganic sheet systems, such as clays self-assembling in layers that form lyotropic lamellar phases in a concentrated regime, but that are able to bend into vesicles in a dilute regime.55
In summary, the most important sodium salt of the anionic icosahedral metallabis(dicarbollide) complexes was the di-iodinated Na[3] because this compound showed an increased antimicrobial effect against all the strains tested in comparison with the parent Na[1]. Other compounds with similar antimicrobial power were Na[6] for Gram-positive bacteria, and Na[5] for Candida sp. Moreover, we could observe that Gram-negative bacteria and Candida albicans have often a similar pattern of response against the different variations of the compounds, which is different from that of Gram-positive bacteria. Probably this is due to the difference in structure and composition of their cell walls. We hypothesize that the outer membrane of Gram-negative bacteria constitutes an impermeable barrier for the majority of these compounds, and that variants such as Na[3] and Na[5], both transoid conformers, represent structures with particular physical–chemical properties that make these compounds more permeable for crossing this barrier.
To shed light upon this hypothesis, we performed two experiments schematically displayed at the left of Scheme 2.
First, we studied the antimicrobial effect of Na[1] on a Gram-negative bacterial model with a mutated lipopolysaccharide (LPS), in particular the Salmonella enterica serotype minnesota Re 595 (Re mutant). LPS is the major component of the outer leaflet of the outer membrane of Gram-negative bacteria, which constitutes an essential structure for cell viability and prevents charged macromolecules and hydrophobic molecules entering the cell.56 LPS are amphiphilic molecules consisting of a glycolipid portion named lipid A, which is embedded in the lipid bilayer of the outer membrane, and a saccharide portion covalently linked to the lipid A that is external. In turn, the saccharide portion consists of two main parts: (i) the core oligosaccharide, which is linked to the lipid A and is formed by sugars that are conserved between species, the first residue being the acidic sugar termed Kdo (3-deoxy-α-D-manno-oct-2-ulopyranosonic acid); and (ii) the more external polysaccharide chain named the O-chain, whose composition is variable within species. Mutants lacking the O-chain have a rough colony aspect in comparison with wild type strains that are smooth. Re mutants lack not only the O-antigen but also part of the core polysaccharide. They are formed by the lipid A and the Kdo, representing the deep-rough chemotype. Moreover, adjacent anionic LPS molecules are linked to divalent cations, mainly Mg2+ and Ca2+, forming a compact surface and making the outer membrane more rigid than normal lipid bilayers.57 We confirmed that these structures are essential for antimicrobial resistance in Gram-negative bacteria since the Na[1] MIC values of the Re 595 mutant decreased to 0.041 μM, and the MBC to 0.082 μM, thus having a sensibility identical to the tested Gram-positive bacteria, while the MIC was 0.65 μM for all the Gram-negative bacteria (Fig. 10).
Second, we performed an experiment combining Na[1] with ethylenediaminetetraacetic acid (EDTA). EDTA is a chelating agent that forms a complex with divalent calcium and magnesium cations present in the outer membrane of Gram-negative bacteria, destabilizing it and thus permeabilizing it.57 A checkerboard dilution method (see Experimental section) was carried out to unambiguously identify whether there is a synergy or additive effect between Na[1] and EDTA or not. For this analysis, Escherichia coli BLEE strain 192348, Salmonella minnesota strain Re 595 (Re mutant) and Staphylococcus aureus MRSA strain 18285 were used as models of a Gram-negative bacterium with intact LPS, a Gram-negative bacterium with mutated LPS, and a Gram-positive bacterium (all lacking an outer membrane), respectively. The MIC of EDTA for these strains was 7 μM, 0.22 μM, and 0.22 μM, respectively.
Fractional inhibitory concentration index (FICI) values (Table 5) and isobolograms (Fig. 11) clearly showed that there is a synergistic or additive effect between Na[1] and EDTA for the Gram-negative E. coli BLEE strain 192348 since, when these two compounds are used in combination, both MIC values are decreased. In contrast, no effect was observed for the Gram-positive Staphylococcus aureus MRSA strain 18285, and the same occurred for the LPS-mutated Salmonella minnesota strain Re 595 (Re mutant). These results strongly suggest that the LPS of the Gram-negative outer membrane plays a crucial role in the natural resistance of Gram-negative bacteria towards Na[1] and other related compounds.
| Strain | MIC Na[1] (μM) | MIC EDTA (μM) | FICI | Interpretation |
|---|---|---|---|---|
| E. coli BLEE strain 192348 | 0.653 | 0.000 | — | — |
| 0.327 | 0.109 | 0.516 | Additivity | |
| 0.163 | 0.219 | 0.281 | Synergy | |
| 0.041 | 0.438 | 0.125 | Synergy | |
| 0.041 | 0.876 | 0.188 | Synergy | |
| 0.041 | 1.752 | 0.313 | Synergy | |
| 0.041 | 3.504 | 0.563 | Additivity | |
| 0.000 | 7.008 | — | — | |
| Salmonella strain Re 595 and S. aureus MRSA strain 18285 | 0.041 | 0.000 | — | — |
| 0.041 | 0.007 | 1.031 | Indifference | |
| 0.041 | 0.014 | 1.063 | Indifference | |
| 0.041 | 0.027 | 1.125 | Indifference | |
| 0.041 | 0.055 | 1.25 | Indifference | |
| 0.041 | 0.109 | 1.5 | Indifference | |
| 0.000 | 0.219 | — | — | |
We have demonstrated that the outer membrane of Gram-negative bacteria constitutes an impermeable barrier for the majority of these compounds, and that variants such as the derivatives [3]− and [5]−, both transoid conformers, in contrast to [1]−, which is cisoid, represent structures with particular physical–chemical properties that make the compounds more permeable for crossing this barrier. While the data are still too sparse for any specific structure–activity relationship evaluation, we believe this study can serve as an important roadmap for additional studies to gain a more in-depth understanding of the mechanism of action of these metallabis(dicarbollides).
The Na[1] cytotoxicity was tested on U87 and T98G glioblastoma cells, A375 melanoma cells and non-tumoral V79 fibroblasts,16 and the cytotoxicity of Na[3] was tested against U87 and T98G glioblastoma cells.44 The IC50 (compound concentration causing 50% inhibition of cell growth) is a parameter used to evaluate the cytotoxicity of a given compound. The IC50 values at 24 h of incubation time with Na[1] and/or Na[3] ranged from 59 μM to 195 μM, displaying no relevant cytotoxic activity, whereas the MIC values for the microorganisms tested here ranged from 0.002 μM to 0.653 μM. The ratio IC50/MIC has been used to calculate the selectivity index (SI) for antimicrobial compounds,58 and it is considered that an SI ≥ 10 is acceptable for a selective bioactive sample.59 The ideal drug should be biologically active at a very low concentration and toxic only at a very high concentration. Taking as a reference the lowest IC50 and the highest MIC for each compound (the worst situation), the SI for both compounds is very high: Na[1] = 165 (IC50 for V79 cells: 102 μM/MIC for Gram-negative: 0.653 μM), and Na[3] = 1180 (IC50 for T98G cells: 59 μM/MIC for Gram-negative: 0.05 μM). The high value of the selectivity indices for Na[1] and Na[3] indicate that these compounds may be useful in managing bacterial and fungal infections.
The fact that these small anionic molecules can cross the mammalian membrane and have antimicrobial properties but low toxicity for mammalian cells represents a promising tool to treat infectious intracellular bacteria. Future experiments determining the effectivity of these compounds during in vitro infection will shed light on this question.
Conclusions
We have reported here the synthesis of several sodium salts of the small anionic metallabis(dicarbollide) molecules ([M(C2B9H11−nXn)2]−, M = Co3+, Fe3+, n = 1, X = H or I): Na[4], Na[5] and Na[6] and their full characterization by FTIR, 1H, 1H{11B}, 11B, 11B{1H} NMR, UV-vis, and MALDI-TOF-MS spectroscopy. The solubility and lipophilicity of the sodium salts of the parent anionic small metallabis(dicarbollide) molecules ([1]−, [2]−, [3]−, [4]−, [5]− and [6]−), which are very important for understanding their biological behavior, are reported. The meta-isomers (Na[5] and Na[6]) display the highest aqueous solubility while the 8,8′-I2-o-metallabis(dicarbollide) isomers (Na[3] and Na[4]) display the lowest. Aggregate formation of the four ortho metallabis(dicarbollides) (Na[1], Na[2], Na[3] and Na[4]) in aqueous solution was studied using DLS, TEM and CryoTEM while no aggregates were observed by DLS and 1H{11B}-NMR for the meta-isomers of the metallabis(dicarbollides). The redox behavior of the sodium salt of the six complexes, corresponding to the redox couples M3+/2+, has been studied by mean of CV, observing reversible processes in all cases. In general, ortho- and meta-Co3+ small molecules are more reducing than the corresponding Fe3+ ones, but their iodine derivatives are less reducing in both cases. The ortho-isomer, [1]−, is more reducing than the meta-isomer[5]−; however the opposite effect is observed in the case of the Fe3+ complexes.The X-ray structures of the protonated salt of the [5]−, [(H3O)(H2O)5][2,2′-Co(1,7-C2B9H11)2], and the cesium salt of the [3]−, [Cs(MeCN)][8,8′-I2-Fe(1,2 C2B9H10)2], are reported. Both crystal structures display transoid conformation of the [M(C2B9H11)2]− cluster. The supramolecular structure of the protonated salt of [5]− presents 2D layers leading to a lamellar arrangement of the small anion molecules, while the cationic part is formed of polymeric water rings made of six- and four-membered rings of water molecules connected via OH⋯H hydrogen bonds. The water layers with weak B–H⋯O contacts help to connect the cationic and anionic layers.
We have demonstrated an antimicrobial effect of these small metallabis(dicarbollide) anionic molecules against Candida species, and against Gram-positive and Gram-negative bacteria, including multiresistant strains. The selectivity index for antimicrobial activity of the compounds Na[1] and Na[3] is very high (165 and 1180, respectively) indicating these compounds may be useful in managing bacterial and fungal infections. This article reveals that small anionic metallabis(dicarbollide) molecules with activities down to the nanomolar range against methicillin resistant S. aureus (MRSA) and a high selectivity index are promising antimicrobial agents to tackle antibiotic-resistant bacteria. It remains to be determined whether the compounds’ concentrations needed to cause an antimicrobial effect in vitro would be applicable, and effective, in vivo. Moreover, we have demonstrated that the outer membrane of Gram-negative bacteria constitutes an impermeable barrier for the majority of these compounds. Nonetheless, changes in the structure of the parent molecule Na[1], such as the meta-isomers or the addition of two iodine groups, had an improved effect against Gram-negative bacteria, possibly due to changes in their physicochemical properties in aqueous media that make the meta-isomers and the di-iodinated ortho-small molecules more permeable for crossing this barrier. To emphasize, the most active metallabis(dicarbollide) small molecules are both transoid conformers, in contrast to [1]−, which is cisoid. Since there is an urgent need for antibiotic discovery and development, this study represents a relevant advance in the field.
Experimental section
Materials
The NaCl was purchased from Sigma–Aldrich, whereas the cationic exchanging resin used (Amberlite IR120, H form) was purchased from Acros Organics and the hydrochloric acid (37%) was purchased from Carlo Erba Reagents. Solvents used were from Carlo Erba SDS and purified by distillation from sodium and benzophenone under a nitrogen atmosphere before use. Na[3,3′-Co(1,2-C2B9H11)2]·2.5H2O abbreviated as Na[1],18 Na[3,3′-Co(8-I-1,2-C2B9H10)2]·2.5H2O abbreviated as Na[3],18 and Na[2]·2.5H2O abbreviated as Na[2]37 were synthesized as reported in the literature. [NMe4][2,2′-Co(1,7-C2B9H11)2] abbreviated as [NMe4][5]35 and [NMe4][2,2′-Fe(1,7-C2B9H11)2] abbreviated as [NMe4][6] were synthesised according to the literature with some modifications.60Instrumentation and measurements
FTIR spectra were run using a Shimadzu FTIR-8300 spectrophotometer. The 1H, 1H{11B} NMR (400.13 MHz) and 11B and 11B{1H} NMR (128.37 MHz) spectra were recorded on a Bruker ARX 400 instrument equipped with the appropriate decoupling accessories. All NMR spectra were performed in the indicated deuterated water at 22 °C. The 11B and 11B{1H} NMR chemical shift values were referenced to external BF3·OEt2, while the 1H, 1H{11B} NMR chemical shift values were referenced to SiMe4. Chemical shifts are reported in units of parts per million downfield from the reference. The mass spectra were recorded in the negative ion mode using a Bruker Biflex MALDI-TOF-MS [N2 laser; λexc 337 nm (0.5 ns pulses); voltage ion source 20.00 kV (Uis1) and 17.50 kV (Uis2)].Cyclic voltammogram responses were recorded at a glassy carbon electrode in MeCN of 0.1 M [NnBu4][PF6] as the supporting electrolyte, with a metallabis(dicarbollide) concentration of 5 mM at a scan rate of 50 mV s−1 on a Autolab PGSTAT204 potentiostat, controlled by Nova 2.1.4 software by Metrohm Autolab. The electrochemical cell contained Ag/AgCl/[NBu4]Cl as the reference electrode, a glassy carbon as the working electrode and Pt wire as the counter one. All experiments were performed at room temperature. The solutions were deaerated with analytical grade nitrogen at the start of each experiment to prevent oxygen interference. All the potential values were referred to the Fc+/Fc couple (E1/2 (Fc+/Fc) = 0.64 V vs. the standard hydrogen electrode (SHE)). UV-vis spectra were recorded on a Jasco V-780 spectrophotometer, using 1 cm cuvettes with 0.08 mM of metallabis(dicarbollides) in water. The hydrodynamic diameter of the samples dispersed in water were studied by dynamic light scattering (DLS) in a Zetasizer Nano ZS (Malvern Instruments Ltd) equipped with a He–Ne 633 nm laser using 1 mL of the sample's dispersion in a disposable glass cuvette. For each compound a couple of samples were run, and the measurements were done in triplicate at ambient temperature with multiple sub-runs. The aqueous sample solutions were previously filtered using a syringe filter (PTFE, 0.2 μm pore diameter). Thermogravimetric analyses/differential scanning calorimetry (TGA/DSC) were performed on a Netzsch STA 449 thermal analyzer at a heating rate of 10 °C min−1 under an Ar atmosphere.
Synthesis and characterization of Cs[3,3′-Fe(8-I-1,2-C2B9H10)2], Cs[4]
Cs[4] was synthesized from Cs[2]. 50 mg (0.11 mmol) of Cs[2] was mixed with 49.2 mg (0.22 mmol) of N-iodosuccinimide and dissolved in 20 mL of ethanol. Subsequently, 0.2 mL (2.27 mmol) of CF3SO4H was added drop by drop, then the reaction flask was closed, and lead react at room temperature for 3 hours. Once the reaction was finished, the solvent was removed under reduced pressure and the product was extracted in THF and a saturated solution of NaCl in 0.1 M HCl solution three times; the organic layer was then dried. Finally, the residue was redissolved in water and a saturated solution of CsCl was added, promoting the precipitation of 146 mg (94% yield) of a dark green product Cs[4]. MALDI-TOF-MS: Theor. 572.45 m/z. Found 573.17 m/z. 1H{11B} NMR (400 MHz, CD3COCD3) δ: 123.10, 58.72 (s, B–H), 43.68 (4H, s, Ccluster–H), 6.82, 1.32, −0.96 and −20.77 (s, B–H). 11B NMR (128.37 MHz, CD3COCD3) δ: 121.8 (2B, B(6,6′), B–H), 26.3 (4B, B(5,5′,11,11′), B–H), 8.3 (4B, (B9,9′,12,12′), B–H), −46.8 (2B, B(10,10′), B–H), −334.9 (4B, B(4,4′,7,7′), B–H) and −568.3 (2B, B(8,8′), B–I).Synthesis and characterization of Na[3,3′-Fe(8-I-1,2-C2B9H10)2]·2.5H2O, Na[4]
Na[4] was obtained by means of the cationic exchange resin from Cs[3,3′-Fe(8-I-1,2-C2B9H10)2]. Approximately 2/3 of the volume of the column (30 cm) was filled with the strongly acidic cationic exchange resin (Amberlite IR120, H form). Before starting, the cationic resin was maintained for 24 h in HCl 3 M to hydrate it. Then, 150 mL of a solution of HCl 3 M was slowly passed through the column to load it with H+. To remove the excess of HCl, distilled water was flowed quickly down the column until a neutral pH was reached. When the desired cation was sodium, a solution of NaCl 3 M was passed through the column slowly to exchange H+ by Na+ until a neutral pH was reached. Distilled water was used to rinse the excess of NaCl through the column. To know if NaCl had been removed, 3 drops of a solution of AgNO3 100 mM were added to a small fraction of the solution coming out of the column until a clear solution was observed. Then, 30 mL of acetonitrile/water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) mixture was flowed through the column to set the column's liquid composition. Approximately 200 mg of Cs[4] was dissolved in a minimum volume of acetonitrile/water (50
50) mixture was flowed through the column to set the column's liquid composition. Approximately 200 mg of Cs[4] was dissolved in a minimum volume of acetonitrile/water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and flowed repeatedly (4 times) through the cationic resin. Before collecting the solution containing the ferrabis(dicarbollide), 50 mL of fresh acetonitrile/water (50
50) and flowed repeatedly (4 times) through the cationic resin. Before collecting the solution containing the ferrabis(dicarbollide), 50 mL of fresh acetonitrile/water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) was added to the column. 50 mL were collected in a flask, the solvent was evaporated and the compound was dried under vacuum. FTIR ν(cm−1) = 3586, 3492 (O–Na), 3031, 3017 (Ccluster–H), 2578, 2552, 2528 (B–H), 1688, 1611 (H2O). 1H{11B} NMR (400 MHz, CD3COCD3) δ: 117.79, 56.31 (s, B–H), 42.16 (4H, s, Cc–H), 1.05, −0.77, −6.07 and −20.20 (s, B–H). 11B NMR (128.37 MHz, CD3COCD3) δ: 114.7 (2B, B(6,6′), B–H), 23.8 (4B, B(5,5′,11,11′), B–H), 6.1 (4B, (B9,9′,12,12′), B–H), −47.1 (2B, B(10,10′), B–H), −322.34 (4B, B(4,4′,7,7′), B–H) and −548.91 (2B, B(8,8′), B–I).61 MALDI-TOF-MS: Theor. 572.45 m/z. Found 573.14 (M, 100%) m/z, where M is the molecular weight of the anion [4]−. Solubility of Na[4] = 374 mM. Anal. Calcd for C4H20B18NaFeI4·2.5H2O: C: 7.49, H: 3.98. Found: C: 7.55, H: 3.93. TGA/DSC: two exothermic weight losses of 1.62 and 2.38% until 160 °C, and 18.82 at 570 °C; the residual mass is 77.98% at 800 °C.
50) was added to the column. 50 mL were collected in a flask, the solvent was evaporated and the compound was dried under vacuum. FTIR ν(cm−1) = 3586, 3492 (O–Na), 3031, 3017 (Ccluster–H), 2578, 2552, 2528 (B–H), 1688, 1611 (H2O). 1H{11B} NMR (400 MHz, CD3COCD3) δ: 117.79, 56.31 (s, B–H), 42.16 (4H, s, Cc–H), 1.05, −0.77, −6.07 and −20.20 (s, B–H). 11B NMR (128.37 MHz, CD3COCD3) δ: 114.7 (2B, B(6,6′), B–H), 23.8 (4B, B(5,5′,11,11′), B–H), 6.1 (4B, (B9,9′,12,12′), B–H), −47.1 (2B, B(10,10′), B–H), −322.34 (4B, B(4,4′,7,7′), B–H) and −548.91 (2B, B(8,8′), B–I).61 MALDI-TOF-MS: Theor. 572.45 m/z. Found 573.14 (M, 100%) m/z, where M is the molecular weight of the anion [4]−. Solubility of Na[4] = 374 mM. Anal. Calcd for C4H20B18NaFeI4·2.5H2O: C: 7.49, H: 3.98. Found: C: 7.55, H: 3.93. TGA/DSC: two exothermic weight losses of 1.62 and 2.38% until 160 °C, and 18.82 at 570 °C; the residual mass is 77.98% at 800 °C.
Synthesis and characterization of Na[2,2′-Co(1,7-C2B9H11)2]·2.5H2O, Na[5]
Na[5] was obtained by the cation exchange procedure using [NMe4][5] as the starting material.35b The yellowish ion exchange resin was maintained for 24 h in HCl solution (3 M), and then used to fill approximately 2/3 of a column. A solution of HCl (3 M) was passed through this column until a transparent solution was obtained. The excess of HCL was removed by rinsing the resin using distilled water until a neutral pH was reached. A solution of NaCl (3 M) was passed slowly through the column to change the H+ to Na+. Distilled water was used in order to remove the excess of NaCl. Subsequently, an acetonitrile/water (50/50) mixture was flowed through the column, and the minimum amount of the same solution was used to dissolve the Cs[5]. This orange solution was passed several times through the column, and then collected, evaporated and dried in vacuum. FTIR ν(cm−1) = 3595, 3374(O–Na), 3039 (Cc–H), 2539 (B–H), 1610 (H2O). 1H{11B} NMR (400 MHz, D2O) δ: 3.55 (s, B–H), 2.97 (4H, s, Cc–H), 2.88, 1.64 (s, B–H). 1H NMR (400 MHz, D2O) δ = 2.96 (4H, s, Ccluster–H). 1H{11B} NMR (400 MHz, CD3COCD3) δ: 3.54, 3.02 (s, B–H). 2.97 (4H, s, Ccluster–H), 1.85, 1.72 (s, B–H). 1H NMR (400 MHz, CD3COCD3) δ: 2.97 (4H, s, Ccluster–H). 11B NMR (128.37 MHz, CD3COCD3) δ: 1.2 (2B, d, 1J(B,H) 141, B–H), −2.3 (4B, d, 1J(B,H) 152, B–H), −9.2 (2B, d, 1J(B–H) 160, B–H), −12.1 (6B, d, 1J(B,H) 150, B–H), −17.7 (4B, d, 1J(B–H) 159, B–H). 11B NMR (128.37 MHz, CD3COCD3) δ: 1.2 (2B, d, 1J(B,H) 141, B–H), −2.3 (4B, d, 1J(B,H) 152, B–H), −9.2 (2B, d, 1J(B–H) 160, B–H), −12.1 (6B, d, 1J(B,H) 150, B–H), −17.7 (4B, d, 1J(B–H) 159, B–H). MALDI-TOF-MS: Theor. 323.75 m/z. Found 324.38 (M, 100%) m/z, where M is the molecular weight of the anion [5]−. Solubility of Na[5] = 1726 mM.Synthesis and characterization of Na[2,2′-Fe(1,7-C2B9H11)2], Na[6]
The same procedure and amounts as those for Na[5] were used, but starting from [NMe4][6] to obtain the Na[6]. 1H{11B} NMR (400 MHz, CD3COCD3) δ: 66.80 (s, B–H), 53.76 (4H, s, Ccluster–H), 6.74, 3.86, 1.19, 0.80, and −0.11 (s, B–H). 11B NMR (128.37 MHz, CD3COCD3) δ: 33.77 (2B, B–H), 26.61(2B, B–H), 10.15 (2B, B–H), −4.49 (2B, B–H), −21.44 (2B, B–H), −35.32 (2B, B–H), −328.78(4B, B(6,6′,11,11′), B–H) and −408.45 (2B, B(9,9′), B–H). MALDI-TOF-MS: Theor. 320.66 m/z. Found 321.38 m/z. Solubility of Na[6] = 1400 mM.Single X-ray diffraction studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 669 reflections to a maximum θ angle of 27.50° (0.77 Å resolution), of which 1284 were independent (average redundancy 20.770, completeness = 99.8%, Rint = 4.52%, Rsig = 1.45%), and 1228 (95.64%) were greater than 2σ(F2). The final cell constants of a = 8.0526(3) Å, b = 11.2324(5) Å, c = 12.1312(6) Å, β = 103.702(2)°, volume = 1066.04(8) Å3, are based upon the refinement of the XYZ-centroids of 88 reflections above 20σ(I) with 6.537° < 2θ < 45.04°. Data were corrected for absorption effects using the multi-scan method (SADABS). The ratio of minimum to maximum apparent transmission was 0.938. The calculated minimum and maximum transmission coefficients (based on crystal size) are 0.8530 and 0.8860.
669 reflections to a maximum θ angle of 27.50° (0.77 Å resolution), of which 1284 were independent (average redundancy 20.770, completeness = 99.8%, Rint = 4.52%, Rsig = 1.45%), and 1228 (95.64%) were greater than 2σ(F2). The final cell constants of a = 8.0526(3) Å, b = 11.2324(5) Å, c = 12.1312(6) Å, β = 103.702(2)°, volume = 1066.04(8) Å3, are based upon the refinement of the XYZ-centroids of 88 reflections above 20σ(I) with 6.537° < 2θ < 45.04°. Data were corrected for absorption effects using the multi-scan method (SADABS). The ratio of minimum to maximum apparent transmission was 0.938. The calculated minimum and maximum transmission coefficients (based on crystal size) are 0.8530 and 0.8860.
The structure was solved and refined using the Bruker SHELXTL software package,62 using the space group C12/m1, with Z = 2 for the formula unit, C4H35B18CoO6. The final anisotropic full-matrix least-squares refinement on F2 with 80 variables converged at R1 = 2.73% for the observed data, and wR2 = 7.52% for all data. The goodness-of-fit was 1.202. The largest peak in the final difference electron density synthesis was 0.354 e− Å−3 and the largest hole was −0.291 e− Å−3 with an RMS deviation of 0.059 e− Å−3. On the basis of the final model, the calculated density was 1.345 g cm−3 and F(000), 446 e−.
X-ray structure determinations of Cs[4], [Cs(MeCN)][4]
The measured crystals were prepared under inert conditions immersed in perfluoropolyether as a protecting oil for the manipulation. Suitable crystals were mounted on MiTeGen MicroMounts and used for data collection. Crystallographic data for Cs[4] were collected at 100 K with a XALOC beamline at the ALBA Synchrotron (λ = 0.82652 Å). Crystallographic data for compound Cs[4] were collected with a Bruker D8 Venture diffractometer. Data were processed with the APEX3 program,63 and corrected for absorption using SADABS.64 The structure was solved by direct methods and subsequently refined by correction of F2 against all reflections62 and Olex2 as the graphical interface.65 All non-hydrogen atoms were refined with anisotropic thermal parameters by full-matrix least-squares calculations on F2. All hydrogen atoms were located in difference Fourier maps and included as fixed contributions riding on attached atoms with isotropic thermal displacement parameters 1.2 or 1.5 (-methyl) times those of the respective atom.A summary of the crystal data is reported in the ESI (Table S1†). CCDC 2149703 for [(H3O)(H2O)5][2,2′-Co(1,7-C2B9H11)2], H[5]) and 2149633 (for [Cs(MeCN)][4] present the crystallographic data of these new crystal structures.†
Microorganisms and growth media
Clinical bacterial (N = 9) and yeast (N = 3) isolates previously tested for susceptibility to other metallacarborane compounds were used here.54 As Gram-negative bacteria, three strains of Escherichia coli and two of Pseudomonas aeruginosa were tested, including strains that are susceptible to commonly used antimicrobials (E. coli 190940 and P. aeruginosa 190089) or are multidrug resistant (E. coli BLEE 192348, E. coli LF82 and P. aeruginosa IMI 187182). As Gram-positive bacteria, we tested two Enterococcus faecalis strains (E. faecalis 190093 and E. faecalis 194844), and two of Staphylococcus aureus (S. aureus MRSA 182851, which is multidrug-resistant, and S. aureus 180895). Yeast strains belonged to the species Candida albicans (C. albicans 180228, C. albicans 181721 and C. albicans 191026). Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 and Salmonella minnesota strain Re 595 (Re mutant) ATCC® 49284 ™, were used as controls to test the effect of the outer membrane lipopolysaccharide on bacterial susceptibility to the compounds.Luria–Bertani (LB) broth was used for growing the E. coli and Salmonella species, brain heart infusion (BHI) for P. aeruginosa, E. faecalis, and S. aureus, and brain heart agar (BHA) for C. albicans.
Determination of minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC)
The MIC was determined by broth microdilution following standardized guidelines,66 and the MBC by counting viable cells at MIC, MIC × 2 and MIC × 4 concentrations following the CLSI standard protocols.67Bacteria were grown in LB (E. coli) or BHI (P. aeruginosa, E. faecalis, and S. aureus) for 16–18 h at 37 °C without shaking. The optical density (OD) of overnight bacterial cultures was measured at 620 nm, and bacterial suspensions were prepared at a final concentration of 1 × 106 CFU mL−1 in cation-adjusted Mueller–Hinton (MH) broth using this relation: OD620 nm of 0.1 ≈ 1.6 × 108 CFU mL−1. Candida inoculums were prepared by resuspending five individual colonies in at least 3 mL of sterile water. The optical density was measured at 530 nm, and suspensions were prepared in sterile water at a final concentration of (1–5) × 105 CFU mL−1.
The compound stock solutions were prepared at 1024 mg L−1 with sterile Milli-Q water and maintained at −20 or 4 °C for a maximum of 1 month. For the bacterial tests, serial dilutions of the compounds were prepared in cation-adjusted Mueller–Hinton broth in a polystyrene 96-well plate. The concentrations tested were 512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, and 0 mg L−1. Bacteria were inoculated at a final concentration of 5 × 105 CFU mL−1 in a final volume of 100 μL. Microplates were incubated for 18 h at 37 °C under aerobic conditions.
The medium used to test Candida was RPMI with 2% glucose and L-glutamine, and the concentrations of the metallabis(dicarbollide) compounds tested were 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, and 0 mg L−1. The test was done in a final volume of 200 μL with (1–5) × 105 CFU mL−1 per well.
Microplates were incubated for 24 h at 37 °C under aerobic conditions. All tests were done in triplicate.
The MIC was determined as the lowest concentration of the compound (indicated in μM) needed to inhibit the growth of the microorganism, which was observed by the absence of visible growth in the well. The MBC was determined by plating 20 μL of the suspension corresponding to the MIC and the two subsequent concentrations tested (MIC × 2 and MIC × 4) onto LB agar plates that did not contain the compound. The MBC was determined as the lowest concentration of compound that reduced the viability of the initial bacterial inoculum at ≥99.9%.
Checkerboard dilution and isobologram
Na[1] was tested in combination with EDTA, an outer membrane permeabilizer, in order to evaluate the impact of the outer membrane on Na[1] ineffectiveness in Gram-negative bacteria. To do so, a checkerboard dilution method was carried out.68 Briefly, Na[1] was serially diluted in MHB along the abscissa of 96-well plates, while EDTA was diluted along the ordinate. The initial concentration for both compounds was the MIC for each bacterium, and it was further two-fold diluted. Each well was inoculated with a bacterial inoculum of 1 × 106 cfu ml−1, with a final concentration of 5 × 105 cfu ml−1 in a final volume of 200 μl. Plates were incubated aerobically at 37 °C for 18 ± 2 h. All the experiments were realized in triplicate.To check the combinatorial effect of the compounds, the fractional inhibitory concentration index (FICI) was calculated as follows: FICI = (MIC EDTA in combination/MIC EDTA) + (MIC Na[1] in combination/MIC Na[1]). The combination is considered synergistic when FIC ≤ 0.5, additive when 0.5 ≤ FICI ≤ 1, indifferent when 1 ≤ FICI ≤ 4, and antagonistic when FICI > 4.68
An isobologram represents the results of the checkerboard assay and the FICI values. The x-axis represents Na[1] and the y-axis EDTA. The line connecting the MIC value of both compounds is the line of indifference (no interaction). Below the line of indifference are additive (1 > FICI > 0.5) and synergistic (FICI ≤ 0.5) interactions. Above the line are indifferent (1 < FICI < 4) and antagonistic (FICI ≥ 4) interactions.69
Author contributions
Clara Viñas: conceived and supervised the study. Margarita Martinez-Medina: designed and supervised the biological studies. Ines Bennour, Ana B. Buades: synthesis and characterization of metallabis(dicarbollides). M. Núria Ramos: biological studies. Miquel Nuez, Jewel Ann Maria Xavier: physico-chemical studies. Duane Choquesillo-Lazarte: X-ray structure measurement. Reijo Sillanpää: discussion and writing results of X-ray structures. Francesc Teixidor and Isabel Romero: discussion and writing results of electrochemistry and UV-visible studies. Clara Viñas, Margarita Martinez-Medina: discussions of the research, writing-review and editing. All authors have read and agreed to the submitted version of the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the Spanish Ministerio de Economía y Competitividad (PID2019-106832RB-100, and SAF2017-82261-P grant cofounded by the European Regional Development Fund) and the Generalitat de Catalunya (2017SGR1720). J. A. M. Xavier acknowledges DOC-FAM program under the Marie Sklodowska-Curie grant agreement N°754397. A. B. Buades, M. Nuez and J. A. M. Xavier are enrolled in the PhD program of the UAB.References
- https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance .
- S. S. Magill, J. R. Edwards, W. Bamberg, Z. G. Beldavs, G. Dumyati, M. A. Kainer, R. Lynfield, M. Maloney, L. McAllister-Hollod, J. Nadle, S. M. Ray, D. L. Thompson, L. E. Wilson and S. K. Fridkin, Multistate point-prevalence survey of health care-associated infections. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team, N. Engl. J. Med., 2014, 370(13), 1198–1208 CrossRef CAS PubMed.
- S. Santajit and N. Indrawattana, Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens, BioMed Res. Int., 2016, 2475067 Search PubMed.
- (a) X. Gu, M. Qiu, H. Sun, J. Zhang, L. Cheng, C. Deng and Z. Zhong, Polytyrosine nanoparticles enable ultra-high loading of doxorubicin and rapid enzyme-responsive drug release, Biomater. Sci., 2018, 6, 1526–1534 RSC; (b) H. Han, Y. Hou, X. Chen, P. Zhang, M. Kang, Q. Jin, J. Ji and M. Gao, Metformin-Induced Stromal Depletion to Enhance the Penetration of Gemcitabine-Loaded Magnetic Nanoparticles for Pancreatic Cancer Targeted Therapy, J. Am. Chem. Soc., 2020, 142, 4944–4954 CrossRef CAS PubMed; (c) Q. Jin, Y. Deng, X. Chen and J. Ji, Rational Design of Cancer Nanomedicine for Simultaneous Stealth Surface and Enhanced Cellular Uptake, ACS Nano, 2019, 13, 954–977 CAS; (d) N. Fernandes, C. F. Rodrigues, A. F. Moreira and I. J. Correia, Overview of the application of inorganic nanomaterials in cancer photothermal therapy, Biomater. Sci., 2020, 8, 2990–3020 RSC; (e) Q. Wang, N. Jiang, B. Fu, F. Huang and J. Liu, Self-assembling peptide-based nanodrug delivery systems, Biomater. Sci., 2019, 7, 4888–4911 RSC; (f) Y. Deng, P. Song, X. Chen, Y. Huang, L. Hong, Q. Jin and J. Ji, 3-Bromopyruvate-Conjugated Nanoplatform-Induced Pro-Death Autophagy for Enhanced Photodynamic Therapy against Hypoxic Tumor, ACS Nano, 2020, 14, 9711–9727 CrossRef CAS PubMed; (g) D. Sun, S. Zhou and W. Gei, What went wrong with anticancer nanomedicine design and how to make it right, ACS Nano, 2020, 14, 12281–12290 CrossRef CAS PubMed.
- (a) Y. Liu, L. Shi, L. Su, H. C. van der Mei, P. C. Jutte, Y. Ren and H. J. Busscher, Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control, Chem. Soc. Rev., 2019, 48, 428–446 RSC; (b) M. Chai, Y. Gao, J. Liu, Y. Deng, D. Hu, Q. Jin and J. Ji, Polymyxin B-Polysaccharide Polyion Nanocomplex with Improved Biocompatibility and Unaffected Antibacterial Activity for Acute Lung Infection Management, Adv. Healthcare Mater., 2020, 9, 1901542 CrossRef CAS PubMed, (1 of 8). (c) M. A. Hutnick and J. K. Pokorski, Mol. Pharm., 2018, 15, 2910–2921 CrossRef CAS PubMed; (d) A. Gupta, S. Mumtaz, C.-H. Li, I. Hussain and V. M. Rotello, Combatting antibiotic-resistant bacteria using nanomaterials, Chem. Soc. Rev., 2019, 48(2), 415–427 RSC.
- S. Wang, Y. Gao, Q. Jin and J. Ji, Emerging antibacterial nanomedicine for enhanced antibiotic therapy, Biomater. Sci., 2020, 8, 6825–6839 RSC.
- C. Brown, F. Chen, C. G. Dowson, G. Dujardin, N. Jung, A. P. King, A. M. Mansour, M. Massi, J. Moat, H. A. Mohamed, A. K. Renfrew, P. J. Rutledge, P. J. Sadler, M. H. Todd, C. E. Willans, J. J. Wilson, M. A. Cooper and M. A. T. Blaskovich, Metal complexes as a promising source for new antibiotics, Chem. Sci., 2020, 11, 2627–2639 RSC.
- A small molecule within the fields of pharmacology and molecular biology is an “organic compound” with a size on the order of 1 nm and a molecular weight <900 daltons that may regulate a biological process.
- (a) Electron Deficient Boron and Carbon Clusters, ed. G. A. Olah, K. Wade and R. E. Williams, Wiley-Interscience, 1st edn, 1991 Search PubMed; (b) C. E. Housecroft, Boranes and Metalloboranes: Structure, bonding, and reactivity (Ellis Horwood Series in Inorganic Chemistry), Pearson Higher Education, 2nd edn, 1994 Search PubMed; (c) C. E. Housecroft, Metal-Metal Bonded Carbonyl Dimers and Clusters, Oxford University Press, 1st edn, 1996 Search PubMed.
- R. N. Grimes, Carboranes, Elsevier Inc., New York, 3rd edn, 2016 Search PubMed.
- J. Plesek, Chemistry of deltahedral boron compounds and organic chemistry. Principal similarity and principal difference, Chem. Rev., 1996, 90(5), 273–279 CAS.
- (a) F. Issa, M. Kassiou and L. M. Rendina, Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds, Chem. Rev., 2011, 111(9), 5701–5722 CrossRef CAS PubMed; (b) M. Scholz and E. Hey-Hawkins, Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies, Chem. Rev., 2011, 111(9), 7035–7062 CrossRef CAS PubMed; (c) Z. J. Leśnikowski, Challenges and Opportunities for the Application of Boron Clusters in Drug Design, J. Med. Chem., 2016, 59, 7738–7758 CrossRef PubMed; (d) P. Stockmann, M. Gozzi, R. Kuhnert, M. B. Sárosi and E. Hey-Hawkins, New keys for old locks: carborane-containing drugs as platforms for mechanism-based therapies, Chem. Soc. Rev., 2019, 48, 3497–3512 RSC.
- J. Poater, C. Vinas, I. Bennour, S. E. Gordils, M. Sola and F. Teixidor, Too Persistent to Give Up: Aromaticity in Boron Clusters Survives Radical Structural Changes, J. Am. Chem. Soc., 2020, 142(20), 9396–9407 CrossRef CAS PubMed.
- (a) J. Rais and P. Selucky, New Trends in the Separation of Cesium, Strontium, and Transplutonides by Extraction Methods, Nucleon, 1992, 1, 17 Search PubMed; (b) S. D. Reilly, C. F. V. Mason and P. H. Smith, Cobalt(III) Dicarbollide: A Potential 137Cs and 90Sr Waste Extraction Agent, Report LA-11695, Los Alamos National Laboratory, Los Alamos, NM, 1990 Search PubMed.
- M. Tarrés, E. Canetta, E. Paul, J. Forbes, K. Azzouni, C. Viñas, F. Teixidor and A. J. Harwood, Biological interaction of living cells with COSAN-based synthetic vesicles, Sci. Rep., 2015, 5, 7804 CrossRef PubMed.
- I. Fuentes, T. García-Mendiola, S. Sato, M. Pita, H. Nakamura, E. Lorenzo, F. Teixidor, F. Marques and C. Viñas, Metallacarboranes on the Road to Anticancer Therapies: Cellular Uptake, DNA Interaction, and Biological Evaluation of Cobaltabisdicarbollide [COSAN]−, Chem. – Eur. J., 2018, 24, 17239–17254 CrossRef CAS PubMed.
- C. Masalles, S. Borrós, C. Viñas and F. Teixidor, Extraordinary Overoxidation resistance increase in self–doped polypyrroles by using non–conventional low charge-density anions, Adv. Mater., 2002, 14, 826–829 CrossRef CAS.
- A. Zaulet, F. Teixidor, P. Bauduin, O. Diat, P. Hirva, A. Ofori and C. Viñas, Deciphering the role of the cation in anionic cobaltabisdicarbollide clusters, J. Organomet. Chem., 2018, 865, 214–225 CrossRef CAS.
- D. C. Malaspina, C. Viñas, F. Teixidor and J. Faraudo, Atomistic simulations of COSAN: amphiphiles without a Head-and-Tail design have a “head and tail” surfactant behavior, Angew. Chem., Int. Ed., 2020, 59, 3088–3092 CrossRef CAS PubMed.
- (a) A. M. A. Abdelgawwad, J. A. M. Xavier, D. Roca-Sanjuán, C. Viñas, F. Teixidor and A. Francés-Monerris, Light-Induced On/Off Switching of the Surfactant Character of the o-Cobaltabis(dicarbollide) Anion with No Covalent Bond Alteration, Angew. Chem., Int. Ed., 2021, 60, 25753–25757 CrossRef CAS PubMed; (b) P. Matejícek, P. Cígler, K. Prochazka and V. Kral, Molecular assembly of metallacarboranes in water: light scattering and microscopy study, Langmuir, 2006, 22, 575–581 CrossRef PubMed; (c) P. Bauduin, S. Prevost, P. Farras, F. Teixidor, O. Diat and T. Zemb, A Theta-Shaped Amphiphilic Cobaltabisdicarbollide Anion: Transition From Monolayer Vesicles to Micelles, Angew. Chem., Int. Ed., 2011, 50, 5298–5300 CrossRef CAS PubMed.
- C. Viñas, M. Tarrés, P. González-Cardoso, P. Farràs, P. Bauduin and F. Teixidor, Surfactant behaviour of metallacarboranes. A study based on the electrolysis of water, Dalton Trans., 2014, 43, 5062–5068 RSC.
- (a) A. I. Stoica, C. Viñas and F. Teixidor, Cobaltabisdicarbollide anion receptor for enantiomer-selective membrane electrodes, Chem. Commun., 2009, 33, 4988–4990 RSC; (b) A. I. Stoica, C. Viñas and F. Teixidor, Application of the cobaltabisdicarbollide anion to the development of ion selective PVC membrane electrodes for tuberculosis drug analysis, Chem. Commun., 2008, 48, 6492–6494 RSC.
- (a) I. Fuentes, J. Pujols, C. Viñas, S. Ventura and F. Teixidor, Dual Binding Mode of Metallacarborane Produces a Robust Shield on Proteins, Chem. – Eur. J., 2019, 25, 12820–12829 CrossRef CAS PubMed; (b) T. M. Goszczynski, K. Fink, K. Kowalski, Z. J. Lesnikowski and J. Boratynski, Interactions of Boron Clusters and their Derivatives with Serum Albumin, Sci. Rep., 2017, 7, 9800 CrossRef PubMed.
- C. Verdiá-Báguena, A. Alcaraz, V. M. Aguilella, A. M. Cioran, S. Tachikawa, H. Nakamura, F. Teixidor and C. Viñas, Amphiphilic COSAN and I2-COSAN crossing synthetic lipid membranes: planar bilayers and liposomes, Chem. Commun., 2014, 50, 6700–6703 RSC.
- (a) R. A. Spryshkova, L. I. Karaseva, V. A. Brattsev and N. G. Serebryakov, Toxicity of functional derivatives of polyhedral carboranes, Med. Radiol., 1981, 26, 62–64 CAS; (b) R. A. Spryshkova, V. A. Brattsev, T. L. Sherman and V. I. Stanko, Accumulation of carborane compounds in the tissues of some animals during Neutron Capture Therapy, Med. Radiol., 1981, 26, 51–55 CAS.
- M. Tarrés, E. Canetta, C. Viñas, F. Teixidor and A. J. Harwood, Imaging in living cells using νB–H Raman spectroscopy: monitoring COSAN uptake, Chem. Commun., 2014, 50, 3370–3372 RSC.
- (a) O. N. Kazheva, G. G. Alexandrov, A. V. Kravchenko, V. A. Starodub, I. A. Lobanova, I. B. Sivaev, V. I. Bregadze, L. V. Titov, L. I. Buravov and O. A. Dyachenko, Molecular conductors with 8,8′-diiodo cobalt bis(dicarbollide) anion, J. Organomet. Chem., 2009, 694, 2336–2342 CrossRef CAS; (b) L. I. Zakharkin, V. A. Ol'shevskaya, E. V. Balagurova and P. V. Petrovskii, Palladium-catalyzed cross coupling of the bis(9-iodo-1,2-dicarbollyl)cobaltate anion with organic magnesium and zinc compounds, yielding bis[9-alkyl(aryl)-1,2-dicarbollyl]cobaltate anions, Russ. J. Gen. Chem., 2000, 70, 550–551 CAS; (c) O. N. Kazheva, G. G. Aleksandrov, A. V. Kravchenko, V. A. Starodub, G. G. Zhigareva, I. B. Sivaev, V. I. Bregadze, L. I. Buravov, L. V. Titov and O. A. Dyachenko, Synthesis, structures, and conductivities of salts (BEDT-TTF)[9,9 ‘(12 ‘)-I-2-3,3 ‘-Co(1,2-C2B9H10)(2)] and (TTF)[9,9 ‘,12,12 ‘-I-4-3,3 ‘-Co(1,2-C2B9H9)(2)], Russ. Chem. Bull., 2010, 59, 1137–1144 CrossRef CAS.
- (a) P. K. Hurlburt, R. L. Miller, K. D. Abney, T. M. Foreman, R. J. Butcher and S. A. Kinkhead, New synthetic routes to B-halogenated derivatives of cobalt dicarbollide, Inorg. Chem., 1995, 34, 5215–5219 CrossRef CAS; (b) L. Matel, F. Macasek, P. Rajec, S. Hermanek and J. Plesek, B-Halogen derivatives of the bis(1,2-dicarbollyl)cobalt(III) anion, Polyhedron, 1982, 1, 511–519 CrossRef CAS; (c) J. Rais, J. Plesek, P. Selucky, M. Kyrs and L. Kadlecova, Extraction of cesium with derivatives of carborane into nitrobenzene, J. Radioanal. Nucl. Chem., 1991, 148(2), 349–357 CrossRef CAS; (d) P. Selucky, J. Rais, M. Kyrs and L. Kadlecova, Extraction of fission-products with 1,2-dichloroethane solutions of hexabromo derivative of cobalt dicarbollide from nitric-acid medium, J. Radioanal. Nucl. Chem., 1991, 148(2), 227–233 CrossRef CAS.
- A. Pepiol, F. Teixidor, R. Sillanpää, M. Lupu and C. Viñas, Stepwise Sequential Redox Potential Modulation Possible on a Single Platform, Angew. Chem., Int. Ed., 2011, 50, 12491–12495 CrossRef CAS PubMed.
- R. Nuñez, I. Romero, F. Teixidor and C. Viñas, Icosahedral boron clusters: a perfect tool for the enhancement of polymer features, Chem. Soc. Rev., 2016, 45, 5147–5173 RSC.
- M. Navascuez, D. Dupin, H.-J. Grand, V. Gómez-Vallejo, I. Loinaz, U. Cosido and J. Llop, COSAN-stabilised omega-3 oil-in-water nanoemulsions to prolong lung residence time for poorly water soluble drugs, Chem. Commun., 2020, 56, 8972–8975 RSC.
- K. Fin and M. Uchman, Boron cluster compounds as new chemical leads for antimicrobial Therapy, Coord. Chem. Rev., 2021, 431, 213684 CrossRef.
- M. J. Macielag, Chemical properties of antibacterials and their uniqueness in Antibiotic Discovery and Development, ed. T. J. Dougherty and M. J. Pucci, Springer US, 2012, pp. 801–802 Search PubMed.
- C. Viñas, F. Teixidor and A. J. Harwood, Cobaltabisdicarbollide-based synthetic vesicles: From biological interaction to in vivo imaging, in Boron-Based Compounds: Potential and Emerging Applications in Medicine, ed. E. Hey-Hawkins and C. Viñas, John Wiley & Sons, 2018, ch. 2.2, pp. 150–171 Search PubMed.
- (a) M. F. Hawthorne, D. C. Young and P. A. Wegner, Carbametallic boron hydride derivatives. I. Apparent analogs of ferrocene and ferricinium ion, J. Am. Chem. Soc., 1965, 87, 1818–1819 CrossRef CAS; (b) M. F. Hawthorne and T. D. Andrews, Carborane analogues of cobalticinium ion, J. Chem. Soc., Chem. Commun., 1965, 443–444 RSC; (c) M. F. Hawthorne, D. C. Young, T. D. Andrews, D. V. Howe, R. L. Pilling, A. D. Pitts, M. Reintjes, L. F. Warren Jr. and P. A. Wegner, pi.-Dicarbollyl derivatives of the transition metals. Metallocene analogs, J. Am. Chem. Soc., 1968, 90, 879–896 CrossRef CAS; (d) C. Viñas, J. Pedrajas, J. Bertran, F. Teixidor, R. Kivekäs and R. Sillanpää, Synthesis of Cobaltabis(dicarbollyl) Complexes Incorporating Exocluster SR Substituents and the Improved Synthesis of [3,3′-Co(1-R-2-R‘-1,2-C2B9H9)2]- Derivatives, Inorg. Chem., 1997, 36, 2482–2486 CrossRef PubMed; (e) I. Bennour, A. Cioran, F. Teixidor and C. Viñas, 3,2,1 and stop! An innovative, straightforward and clean route for the flash synthesis of metallacarboranes, Green Chem., 2019, 21, 1925–1928 RSC.
- T. I. Rokitskaya, I. D. Kosenko, I. B. Sivaev, Y. N. Antonenko and V. I. Bregadze, Fast flip–flop of halogenated cobalt bis(dicarbollide) anion in a lipid bilayer membrane, Phys. Chem. Chem. Phys., 2017, 19, 25122–25128 RSC.
- T. Garcia-Mendiola, V. Bayon-Pizarro, A. Zaulet, I. Fuentes, F. Pariente, F. Teixidor, C. Viñas and E. Lorenzo, Metallacarboranes as tunable redox potential electrochemical indicators for screening of gene mutation, Chem. Sci., 2016, 7, 5786–5797 RSC.
- J. Brus, A. Zhigunov, J. Czernek, L. Kobera, M. Uchman and P. Matejicek, Control over the Self-Assembly and Dynamics of Metallacarborane Nanorotors by the Nature of the Polymer Matrix: A Solid-State NMR Study, Macromolecules, 2014, 47, 6343–6354 CrossRef CAS.
- O. N. Kazheva, A. V. Kravchenko, I. D. Kosenko, G. G. Alexandrov, D. M. Chudak, V. A. Starodub, I. A. Lobanova, V. I. Bregadze, L. I. Buravov, S. G. Protasova and O. A. Dyachenko, J. Organomet. Chem., 2017, 849–850, 261–267 CrossRef CAS.
- (a) CSD consulted in March 15th 2021. ; (b) F. H. Allen, The Cambridge Structural Database: a quarter of a million crystal structures and rising, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef PubMed; (c) I. J. Bruno, J. C. Cole, P. R. Edgington, M. Kessler, C. F. Macrae, P. McCabe, J. Pearson and R. Taylor, New software for searching the Cambridge Structural Database and visualizing crystal structures, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 389–397 CrossRef PubMed.
- D. M. P. Mingos and A. L. Rohl, Size and shape characteristics of inorganic molecules and ions and their relevance to molecular packing problems, J. Chem. Soc., Dalton Trans., 1991, 3419–3425 RSC.
- P. Sivy, A. Preisinger, O. Baumgartner, F. Valach, B. Koren and L. Matel, Structure of caesium 3,3′-commo-bis(8,9,12-tribromooctahydro-1,2-dicarba-3-cobalta-closo-dodecaborate)(1-), Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1986, 42, 24–27 CrossRef.
- (a) G. Chevrot, R. Schurhammer and G. Wipff, Surfactant Behavior of “Ellipsoidal” Dicarbollide Anions: A Molecular Dynamics Study, J. Phys. Chem. B, 2006, 110, 9488–9498 CrossRef CAS PubMed.
- M. Nuez-Martinez, C. I. G. Pinto, J. F. Guerreiro, F. Mendes, F. Marques, A. Muñoz-Juan, J. A.-M. Xavier, A. Laromaine, V. Bitonto, N. Protti, S. Geninatti Crich, F. Teixidor and C. Viñas, Cobaltabis(dicarbollide) ([o-COSAN]−) as Multifunctional Chemotherapeutics: A Prospective Application in Boron Neutron Capture Therapy (BNCT) for Glioblastoma, Cancers, 2021, 13, 6367 CrossRef CAS PubMed.
- M. Corsini, F. Fabrizi de Biani and P. Zanello, Mononuclear metallacarboranes of groups 6–10 metals: Analogues of metallocenes: Electrochemical and X-ray structural aspects, Coord. Chem. Rev., 2006, 250, 1351–1372 CrossRef CAS.
- M. Lupu, A. Zaulet, F. Teixidor, E. Ruiz and C. Viñas, Negatively Charged Metallacarborane Redox Couples with Both Members Stable to Air, Chem. – Eur. J., 2015, 21(18), 6888–6897 CrossRef CAS PubMed.
- I. Guerrero, Z. Kelemen, C. Viñas, I. Romero and F. Teixidor, Metallacarboranes as Photoredox Catalysts in Water, Chem. – Eur. J., 2020, 26, 5027–5036 CrossRef CAS PubMed.
- (a) C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug Delivery Rev., 2001, 46, 3–26 CrossRef CAS; (b) W. L. Jorgensen and E. M. Duffy, Prediction of drug solubility from structure, Adv. Drug Delivery Rev., 2002, 54, 355–366 CrossRef CAS; (c) J. Wang, G. Krudy, T. Hou, W. Zhang, G. Holland and X. Xu, Development of reliable aqueous solubility models and their application in druglike analysis, J. Chem. Inf. Model, 2007, 47, 1395–1404 CrossRef CAS PubMed.
- O. N. Kazheva, G. G. Alexandrov, A. V. Kravchenko, I. B. Sivaev, I. D. Kosenko, I. A. Lobanova, M. Kajnakova, L. I. Buravov, V. I. Bregadze, A. Feher, V. A. V. Starodub and O. A. Dyachenko, Synthesis, structure, electrical and magnetic properties of (BEDT-TTF)2[3,3′-Fe(1,2-C2B9H11)2], Inorg. Chem. Commun., 2012, 15, 106–108 CrossRef CAS.
- The size was measured from a search at the Cambridge Structural Database (CSD) done on January 19th 2022.
- The Partition Coefficient (P) is defined as the ratio of the amount of compound present in the organic phase (n-octanol) to the amount present in the aqueous phase.
- V. W. Pike, PET radiotracers: crossing the blood–brain barrier and surviving metabolism, Trends Pharmacol. Sci., 2009, 30, 431–440 CrossRef CAS PubMed.
- (a) T. Popova, A. Zaulet, F. Teixidor, R. Alexandrova and C. Viñas, Investigations on antimicrobial activity of cobaltabisdicarbollides, J. Organomet. Chem., 2013, 747, 229–234 CrossRef CAS; (b) Y. Zheng, W. W. Liu, Y. Chen, H. Jiang, H. Yan, I. Kosenko, L. Chekulaeva, I. Sivaev, V. Bregadze and X. M. Wang, A Highly potent antibacterial agent targeting methicillin-resistant staphylococcus aureus based on cobalt bis(1,2-dicarbollide) alkoxy derivative, Organometallics, 2017, 36(18), 3484–3490 CrossRef CAS; (c) E. Kvasničková, J. Masák, J. Čejka, O. Matátková and V. Šícha, Preparation, characterization, and the selective antimicrobial activity of N-alkylammonium 8-diethyleneglycol cobalt bis-dicarbollide derivatives, J. Organomet. Chem., 2017, 827, 23–31 CrossRef.
- I. Romero, M. Martinez-Medina, C. Camprubí-Font, I. Bennour, D. Moreno, L. Martínez-Martínez, F. Teixidor, M. A. Fox and C. Viñas, Metallacarborane Assemblies as Effective Antimicrobial Agents, Including a Highly Potent Anti-MRSA Agent, Organometallics, 2020, 39, 4253–4264 CrossRef CAS.
- D. Brusselle, P. Bauduin, L. Girard, A. Zaulet, C. Viñas, F. Teixidor, I. Ly and O. Diat, Lyotropic Lamellar Phase Formed from Monolayered θ-Shaped Carborane-Cage Amphiphiles, Angew. Chem., Int. Ed., 2013, 52, 12114–12118 CrossRef CAS PubMed.
- A. Ebbensgaard, H. Mordhorst, F. M. Aarestrup and E. B. Hansen, The Role of Outer Membrane Proteins and Lipopolysaccharides for the Sensitivity of Escherichia coli to Antimicrobial Peptides, Front. Microbiol., 2018, 9, 2153 CrossRef PubMed.
- M. Vaara, Agents that increase the permeability of the outer membrane, Microbiol. Rev., 1992, 395–411 CrossRef CAS PubMed.
- DOI:10.1016/j.bbrep.2016.03.014 .
- DOI:10.1016/bs.podrm.2020.07.005 .
- I. Bennour, M. Haukka, F. Teixidor, C. Viñas and A. Kabadou, Crystal structure and Hirshfeld surface analysis of [N(CH3)4][2,2′-Fe(1,7-closo-C2B9H11)2], J. Organomet. Chem., 2017, 846, 74–80 CrossRef CAS.
- The chemical shifts of the Boron vertices were assigned according to: A. M. Cioran, F. Teixidor and C. Viñas, The effect of a paramagnetic metal ion within a molecule: comparison of the structurally identical paramagnetic [3,3-Fe(1,2-C2B9H11)2]− with the diamagnetic [3,3-Co(1,2-C2B9H11)2]− sandwich complexes, Dalton Trans., 2015, 44, 2809–2818 RSC.
- G. M. Sheldrick, A short history of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64(1), 112–122 CrossRef CAS PubMed.
- Bruker APEX3 Software, V2019.0-1, Bruker AXS Inc., Madison, Wisconsin, 2019 Search PubMed.
- L. Krause, R. Herbst-Irmer, G. M. Sheldrick and D. Stalke, J. Appl. Crystallogr., 2015, 48, 3–10 CrossRef CAS PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- International Standards Organisation, Clinical laboratory testing and in vitro diagnostic test systems d Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 1: Reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. ISO-20776e1, 2006 Search PubMed.
- CLSI, Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. document M26-A, 1999 Search PubMed.
- R. Hamoud, S. Zimmermann, J. Reichling and M. Wink, Synergistic interactions in two-drug and three-drug combinations (thymol, EDTA and vancomycin) against multi drug resistant bacteria including E. coli, Phytomedicine, 2014, 21(4), 443–447 CrossRef CAS PubMed.
- DOI:10.1016/j.phymed.2013.10.016 .
Footnote |
| † Electronic supplementary information (ESI) available: Scheme S1–S4 displaying the synthetic procedures to obtain Na[1], Na[2], Na[3], Na[5] and Na[6]. Characterization of the compounds, FTIR, 11B{1H}, 1H, 1H{11B}, and 11B NMR, MALDI-TOF-MS, and CV. 1H{11B}, and 11B{1H} NMR studies of the Na[5] and Na[6] at different concentrations. Solubility and lipophilicity studies of the ortho- and meta-metallabis(dicarbollides) as well as the 8,8′-I2-ortho- derivatives. Crystal data and structure refinement of [(H3O)(H2O)5][2,2′-Co(1,7-C2B9H11)2], H[5], and [Cs(MeCN)][8,8′-Fe(8-I-1,2-C2B9H10)2], Cs[4]. CCDC 2149703 and 2149633. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2dt01015a |
| This journal is © The Royal Society of Chemistry 2022 |