Open Access Article
Open Access ArticleA combination of proton spin diffusion NMR and molecular simulations to probe supramolecular assemblies of organic molecules in nanoporous materials†
Eddy
Dib
 *a,
Beatriz
Bernardo-Maestro
b,
Fernando
López-Arbeloa
c,
Joaquín
Pérez-Pariente
*a,
Beatriz
Bernardo-Maestro
b,
Fernando
López-Arbeloa
c,
Joaquín
Pérez-Pariente
 b and
Luis
Gómez-Hortigüela
b and
Luis
Gómez-Hortigüela
 *b
*b
aNormandie Univ, ENSICAEN, UNICAEN, CNRS, Laboratoire Catalyse et Spectrochimie, 14000 Caen, France. E-mail: eddy.dib@ensicaen.fr
bInstituto de Catálisis y Petroleoquímica, ICP-CSIC. C/Marie Curie 2, 28049 Madrid, Spain. E-mail: lhortiguela@icp.csic.es
cDepartamento de Química Física, Universidad del País Vasco, Apartado 644, 48080, Bilbao, Spain
First published on 11th March 2022
Abstract
In this work we show the use of high-resolution 1H MAS NMR to distinguish between two kinds of aggregation states of (1R,2S)-ephedrine, a chiral organic structure directing agent, occluded within AFI-type microporous aluminophosphates. We investigate in particular the supramolecular assembly of the molecules through π⋯π type interactions of their aromatic rings when confined within the one-dimensional AFI channels. A series of high-resolution two-dimensional spin diffusion spectra combined with molecular simulations and DFT calculations allowed us to distinguish different aggregation states of ephedrine molecules and precisely estimate the distances between the aromatic rings and their closest protons inside the zeolite channels as a consequence of distinct proton spin diffusion profiles.
1. Introduction
The development of chiral solid materials able to perform chemical operations in adsorption or catalysis in an enantioselective way is crucial in the chemical industry (especially in the pharmaceutical sector) in order to produce pure enantiomers of chiral compounds.1 Among the different types of active solids, zeolites, which are microporous crystalline materials based on connected SiO4 tetrahedra, have been proposed as excellent candidates for producing chiral-active solid materials as they can potentially combine their unique shape-selectivity capabilities with enantioselective properties.2–4The most straightforward strategy to induce chirality in zeolite frameworks is through the use of chiral organic molecules as structure-directing agents (SDA).5,6 These SDAs organize the inorganic units around in a particular geometry, driving the crystallization pathway towards a particular framework type, remaining occluded within the porous structure after the crystallization process. Therefore, the study at molecular level of the structure-directing effect of chiral organic molecules during the synthesis of zeolite microporous materials is fundamental in order to realize a transfer of chirality from organic species to inorganic zeolite frameworks.7 This has been recently accomplished for the STW chiral framework by using computationally-designed chiral SDAs that follow the helicoidal pattern of the micropores of the STW network.8,9
In an attempt to induce chirality in zeolite frameworks, we proposed the use of the alkaloid (1R,2)-ephedrine (EPH) as chiral SDA (Scheme 1).10 In previous works we showed that these molecules tend to form supramolecular aggregates when confined within the one-dimensional pores of the AFI aluminophosphate framework, in which part of the aluminum atoms of the framework have been isomorphically substituted by Mg2+ cations.11,12 Indeed, the aggregation of these species confined within AFI nanopores (7.3 Å of diameter) can be altered by modifying the crystallization conditions, in particular with gel composition and crystallization temperature:13 high Mg and low SDA contents and low crystallization temperatures favor the incorporation of EPH as monomers (Fig. 1-top), whilst the opposite (low Mg, high SDA and high crystallization temperature) favor the occlusion of EPH as dimers (Fig. 1-bottom). Moreover, the molecular structure of the ephedrine derivative also strongly affects the supramolecular behaviour, with (1R,2S)-ephedrine displaying the strongest self-assembly trend.11,14,15
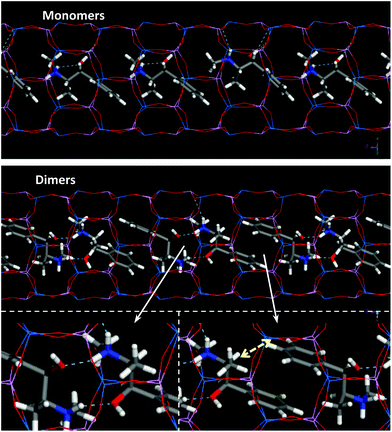 | ||
| Fig. 1 Location of ephedrine molecules in monomer (top) or dimer (bottom) configurations. Yellow arrow indicates short H(para)⋯H3 intermolecular distances (see text below). | ||
As a matter of fact, the development of supramolecular assemblies of organic species as structure-directing entities has become a fundamental concept in zeolite synthesis that has led to the crystallization of a number of open frameworks or new compositions of already known structures.16–25 Under this strategy, which has been referred as supramolecular assembly templating (SAT), simple organic species form supramolecular aggregates, usually through development of π–π type interactions (provided aromatic rings are present), whose larger size tends to drive the crystallization pathway towards large-pore and/or large-cavity-based materials. In this context, the monitoring of the supramolecular behavior of these molecules confined within microporous materials represents a crucial issue. As shown in previous works, UV-VIS fluorescence spectroscopy has proved as an efficient tool to monitor the aggregation state of the molecules confined in nanoporous spaces.17,26 The formation of supramolecular dimers through stacking of the aromatic rings involves strong π–π type interactions that result in a reorganization of the electronic levels, causing a red-shift of the emission band of the aromatic ring.
Solid-state nuclear magnetic resonance (NMR) is a powerful tool to probe non-covalent host–guest interactions in supramolecular assemblies, e.g. hydrogen bonding, van der Waals and π–π interactions, giving unique, atomic-level information about structure and dynamics of guest molecules adsorbed in solid materials.27,28 However, getting precise information about internuclear distances when proton nuclei are involved is hindered by intense dipole–dipole couplings, giving rise to spectral broadenings.29 Highly resolved spectra are usually obtained using magic angle spinning that aims to average dipole–dipole couplings among other anisotropic interactions. Hence, the increase of resolution is on detriment of precious information like internuclear distances. For this reason, several two-dimensional recoupling techniques have been designed to reintroduce the dipole–dipole coupling in one dimension while having a highly resolved spectrum in the other. These techniques are either homonuclear or heteronuclear.30 We have recently localized silanol defects in as-synthesized silicalite-1 using homonuclear 1H–1H DQ-SQ recoupling technique,31 and probed the aluminum distribution in ZSM-5 using heteronuclear 29Si–27Al symmetry-based recoupling technique.32 Moreover, proton spin diffusion (PSD) process describes the magnetization exchange between protons driven by the dipolar coupling dependent on internuclear distances, potentially giving access to information about internuclear distances.33
In this work we use high-resolution 1H NMR measurement of PSD process combined with DFT calculations, to distinguish different supramolecular behaviors of ephedrine molecules when confined within nanopores, getting a precise idea on the intermolecular distances separating the molecules, providing precious information about packing interactions in host–guest systems.
2. Experimental
2.1 Synthesis and general characterization
Synthesis of MgAPO-5 materials using (1R,2S)-ephedrine (EPH) as SDA was carried out following our previously reported recipes.13 The solids were characterized by powder X-ray diffraction (XRD) (Philips X'PERT diffractometer with Cu [Kα] radiation with a Ni filter), thermogravimetric analyses (TGA) (Perkin-Elmer TGA7 instrument [heating rate = 20 °C min−1] under air flow), and UV–Visible diffuse reflectance spectroscopy (UV-Vis Cary 5000 Varian spectrophotometer). Solid state UV-visible fluorescence emission spectra were recorded in a RF-5300 Shimadzu fluorimeter in the front-face configuration.2.2 Nuclear magnetic resonance
Magic-angle spinning (MAS) NMR spectra were acquired at a 11.7 T Bruker Avance III-HD spectrometer, using 1.9 OD-mm probe head for 1H. The rotor was spun with a spinning rate of 40 kHz, a radiofrequency power of 125 kHz was used, and 16 scans were acquired with a recycle delay of 5 seconds. The experimental parameters used for other nuclei are given in the ESI.† The 1H–1H spin diffusion spectra were acquired with short spin diffusion mixing times varying from 0 to 1 millisecond.2.3 Computational details
Plane waves DFT-D theoretical calculations have been performed with the CASTEP module in Material Studio,34 using PBE35 as functional; the Grimme dispersion term36 was used to account for dispersion interactions. The most stable location of ephedrine in different configurations in the AFI systems, with 3 [for monomers] or 4 [for dimers] protonated EPH cations in 1 × 1 × 3 AFI supercells in the required orientations, was found by molecular mechanics-based simulated annealing calculations, using the cvff forcefield.37 The systems were then geometry optimized at DFT-D level. Calculation of the NMR chemical shifts were performed with the gauge-including projector augmented-wave method (GIPAW) developed by Pickard and Mauri.381H chemical shieldings (σ) were converted into chemical shifts (õ) by using reference values of σref = 30, in order to make them roughly coincide with the expected chemical shift values of the aromatic protons (õ = σref − σ); averaged values for the different chemically equivalent protons in the different molecules present in the models will be considered.3. Results and discussion
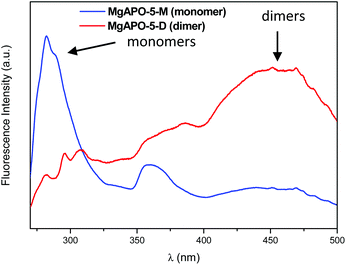
In previous works we showed that EPH tends to direct the crystallization of the AFI framework structure, which is based on one-dimensional 12-MR channels (Fig. 1). Interestingly, we had observed that the supramolecular behavior of this molecule when confined within nanoporous materials can be altered by tuning the synthesis conditions.13 In this work, two MgAPO-5 (AFI-framework type) samples have been studied for which the synthesis conditions were adjusted in order to have different aggregation states of ephedrine molecules, arranged as monomers (M) or dimers (D) (Fig. 1). X-ray diffraction patterns showed that both samples are pure MgAPO-5 materials (Fig. S1†).Depending on the crystallization temperature and the gel composition, two different EPH species in the AFI channels can be spectroscopically identified. The UV-VIS fluorescence spectrum of the sample obtained at 165 °C from gel composition 0.32 MgO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EPH
1 EPH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.84 Al2O3
0.84 Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 P2O5
1 P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 H2O (with high Mg and water contents) consists in an emission peak centered at 282 nm with an overlapped vibrational shoulder at around 295 nm (Fig. 2-blue) which is assigned to the EPH monomer. In contrast, the fluorescence spectrum of the sample obtained upon a reduction of the Mg and water contents (gel composition of 1 EPH
100 H2O (with high Mg and water contents) consists in an emission peak centered at 282 nm with an overlapped vibrational shoulder at around 295 nm (Fig. 2-blue) which is assigned to the EPH monomer. In contrast, the fluorescence spectrum of the sample obtained upon a reduction of the Mg and water contents (gel composition of 1 EPH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.22 MgO
0.22 MgO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.89 Al2O3
0.89 Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 P2O5
1 P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 H2O) and an increase in the crystallization temperature (to 180 °C) presents a main broad emission band in the blue VIS region between 425 and 500 nm (Fig. 2-red) attributed to an aggregate (dimer) state of EPH26 (no higher-order aggregates can be confined within the AFI channels); in this case, π⋯π interactions between stacked aromatic rings composing the dimer involve a reorganization of the electronic levels, promoting a stabilization of the π* molecular orbital which reduces the transition energy, resulting in such shift to higher wavelengths. The formation of monomer and dimer species of EPH in the AFI channels is confirmed by UV/VIS diffuse reflectance spectroscopy (Fig. S2†), with two clear absorption peaks at ∼215 and ∼260 nm (with a vibrational profile) for EPH monomers (Fig. S2†-blue for MgAPO-5-M) and a broad and an additional overlapping absorption band centered at around 360 nm, characteristic of the presence of EPH dimers (Fig. S2†-red for MgAPO-5-D).
50 H2O) and an increase in the crystallization temperature (to 180 °C) presents a main broad emission band in the blue VIS region between 425 and 500 nm (Fig. 2-red) attributed to an aggregate (dimer) state of EPH26 (no higher-order aggregates can be confined within the AFI channels); in this case, π⋯π interactions between stacked aromatic rings composing the dimer involve a reorganization of the electronic levels, promoting a stabilization of the π* molecular orbital which reduces the transition energy, resulting in such shift to higher wavelengths. The formation of monomer and dimer species of EPH in the AFI channels is confirmed by UV/VIS diffuse reflectance spectroscopy (Fig. S2†), with two clear absorption peaks at ∼215 and ∼260 nm (with a vibrational profile) for EPH monomers (Fig. S2†-blue for MgAPO-5-M) and a broad and an additional overlapping absorption band centered at around 360 nm, characteristic of the presence of EPH dimers (Fig. S2†-red for MgAPO-5-D).
Interestingly, TGA results (Fig. S3†) show a higher incorporation of organics in the sample where dimers predominate (MgAPO-5-D, with 1.26 EPH per u.c., compared to MgAPO-5-M, with 1.13 EPH per u.c.). This suggests a more efficient packing of ephedrine molecules in the AFI channels when they arrange as dimers (see Fig. 1). In the MgAPO-5-M sample, the lower organic incorporation is accompanied by a slightly higher water content. SEM images (Fig. S4†) show the occurrence of hexagonal prismatic crystals typical of AFI materials, these crystals being larger in sample MgAPO-5-D, obtained from more concentrated gels. Moreover, SEM also shows the presence of a minor amount of amorphous material in the MgAPO-5-M sample.
13C MAS NMR (Fig. S5†) shows all the typical bands associated to EPH, evidencing the resistance of ephedrine molecules to the hydrothermal treatment and their integral incorporation in both MgAPO-5 materials; broader bands are observed for dimers in MgAPO-5 (Fig. S5,† red line). 27Al MAS NMR spectra (Fig. S6†) show a band at 38 ppm characteristic of tetrahedral aluminum in the AFI framework; a minor presence of penta-coordinated Al at 8 ppm is also observed, more abundant in sample MgAPO-5-M, possibly coming from the amorphous material, as observed by SEM (see Fig. S4†). 31P MAS NMR (Fig. S7†) shows two bands at −30 and −24 ppm, characteristic of P(4Al) and P(1Mg,3Al) environments in the AFI tetrahedral network, respectively,39 confirming the incorporation of Mg atoms in the AFI framework. This incorporation is higher for sample MgAPO-5-D than for MgAPO-5-M (1.30 and 1.03 Mg per u.c., respectively, as obtained from deconvolution of the 31P NMR spectra), despite the higher concentration of Mg in the synthesis gel used for MgAPO-5-M. Indeed, these Mg contents are in line with the EPH contents (as calculated from TGA) since these (protonated) molecules provide charge-balance for the isomorphic substitution of Al3+ by Mg2+. The lower packing efficiency of ephedrine monomers (limited to 1.0–1.1 EPH per u.c.) in this material thus restricts the incorporation of Mg in the framework to 1.1.
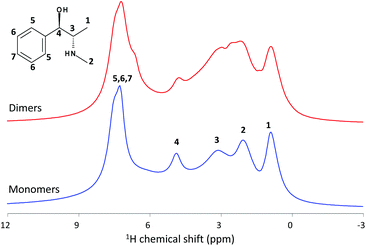
The conventional 1H MAS NMR spectra of the as-synthesized MgAPO-5 materials are shown in Fig. 3. They display a number of resonances between 1 and 8 ppm assigned to ephedrine protons. Four clear NMR signals are observed for EPH monomers at 0.9, 2.1, 3.1 and 4.9 ppm (blue line), which are assigned to protons 1, 2, 3 and 4, respectively, apart from a broad band at 7.3 ppm with several peaks corresponding to aromatic protons. When we compare with the dimer spectrum, several clues reveal the existence of different molecular assemblies in both samples. The first observation is the increase of the peaks’ width in the spectrum of sample MgAPO-5-D, where the observed loss of resolution may be due to (i) the aggregation of the molecules (wider distribution of chemical environments giving rise to wider chemical shift ranges for each proton in the molecule) or (ii) the dipolar coupling between the protons (directly related to the inter-nuclear distances). Indeed, several overlapped peaks in the H2–H3 region are observed, which might be associated to dimers in different orientations. In addition, a notable difference is observed on dimers where a new band arises at 6.7 ppm as a shoulder of the aromatic band, which must be associated to π⋯π stacked aggregates.
In an attempt to understand these differences, we used DFT calculations to predict the chemical shifts as a function of the supramolecular state (Table 1). We focus on aromatic protons which are the ones that will mostly feel the π⋯π stacking interaction upon formation of supramolecular dimers and the ones less affected by the free-movement of protons (considering that these are static DFT calculations); indeed, differences in H2 and H1 predicted shifts (Table 1) might be associated to static DFT calculations artifacts and the presence of different molecular conformations/orientations. Theoretical results show that protons that mostly feel the π⋯π stacking interaction are those in meta and especially in para positions since they are closer to the aromatic ring of the other molecule composing the dimer (see Fig. 1), explaining that these protons show a larger difference in the predicted chemical shift upon aggregation. Interestingly, our modeling results predict a clear shift of the band corresponding to H in para position of the aromatic rings upon formation of dimers towards lower chemical shifts (from 7.4 to 6.5 ppm, Table 1), which must be due to the proximity to the next EPH molecule in the dimer. This observation might explain the shoulder observed in the 1H NMR spectrum of MgAPO-5-D sample.
| H meta | H ortho | H para | CHOH (4) | CHCH3 (3) | CH3N (2) | CHCH3 (1) | |
|---|---|---|---|---|---|---|---|
| M | 7.6 | 7.5 | 7.4 | 5.1 | 2.6 | 1.5 | 0.5 |
| D | 7.0 | 7.1 | 6.5 | 5.0 | 3.0 | 2.9 | 1.4 |
Two-dimensional (2D) spin diffusion 1H MAS NMR allows to get precious information on the internuclear distances from the measurements of the proton spin diffusion (PSD) coefficients.40,41 They are directly related to the internuclear distances when short diffusion periods are used, as relaxation effects can be ignored for short spin diffusion times.42,43 However, the spin diffusion rate depends also on the orientation of the internuclear vector in the solid and the MAS rate, and a phenomenological multi-spin kinetic rate matrix approach was proposed. Thus, the combination of PSD approach with DFT calculations allows an accurate determination of 1H–1H distances.44
Fig. 4 shows two examples of 2D PSD spectra recorded with a mixing time of 0 and 1 milliseconds. The intensity of the peaks varies with the mixing time tSD due to proton spin diffusion; the evolution of the polarization Pij obeys the equation Pij(tSD) = exp[−kij·tSD]ijM0z, with kij being the spin diffusion rate constant between a pair of spins i and j, and M0z the longitudinal magnetization. Then, with increasing spin diffusion time, the intensity of diagonal cross peaks decreases while off-diagonal cross peaks get more intense, as shown in Fig. S9.† Interestingly, the spin diffusion rate varies from one proton to another but interference may occur between several types of intermolecular and intramolecular protons, and it is complex to estimate all distances between all protons, especially inside the channels where a statistical distribution of negative and positive charges occurs with possibly different configurations. Then, we will only consider protons of the phenyl groups, the ones most involved in the π⋯π stacking interactions. It is also important to note that all relaxation processes are neglected at this time-scale and proton–proton spin-diffusion processes are the only ones responsible for the decay or build-up of the diagonal or off-diagonal peaks, respectively.
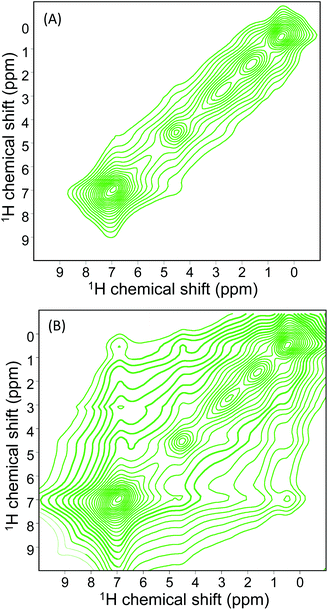 | ||
| Fig. 4 1H NMR spin diffusion spectrum of the sample MgAPO-5-M (monomers) recorded at 500 MHz at two different mixing times 0.0 (A) and 1.0 ms (B). | ||
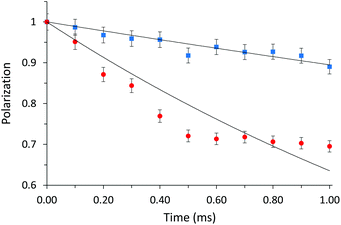
Fig. 5 shows the evolution of the diagonal peak area corresponding to the phenyl groups with spin diffusion time for MgAPO-5-M and -D samples. The spin diffusion rate constant kij is much higher for sample with EPH dimers: 1.1134 ± 0.0636 ms−1 for MgAPO-5-M vs. 4.6841 ± 0.3249 ms−1 for MgAPO-5-D; the high error for the latter indicates the eventual presence of some monomers. These differences are mainly driven by dipolar couplings, spin diffusion processes, occurring either between equivalent or different spins, which depend directly on internuclear distances and, as a consequence, on supramolecular assemblies. They are described using the diffusion equation (vide supra).
Nevertheless, the spin diffusion rate is directly related to 1H–1H distances via the equation kij = A(μ0γH2h/8π)2/rij6, where μ0, γH, and h are physical constants, rij is the internuclear distance between atoms i and j, and A is a phenomenological scaling factor, set to 1016 for both normalized curves. Using this equation, we can estimate average distances separating aromatic rings from their closest protons in both samples, considering the diffusion with the first neighbor spin as the most effective one. These distances correspond to 3.27 and 4.15 Å for MgAPO-5-D and MgAPO-5-M, respectively, being lower for the arrangement of dimers. This proves the higher packing efficiency of EPH when arranged as dimers, which prompts a higher incorporation of organics, as observed from TGA and indirectly also by 31P NMR results. The incorporation of dimers is a consequence of π⋯π interactions between the aromatic rings, but can also be assisted by the potential development of intermolecular H-bonds between consecutive dimers to compensate for the electrostatic repulsion generated by their positive charge associated to N.
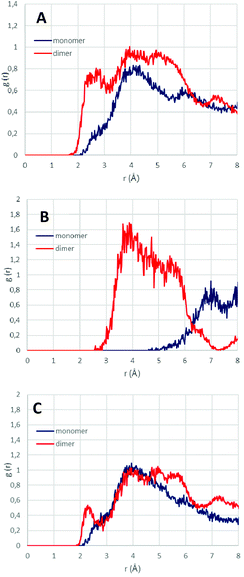
In order to correlate such packing efficiency and the associated higher proton spin diffusion rate with shorter interatomic distances between protons, we ran molecular dynamics simulations (100 ps in the NVT ensemble at 25 °C) for monomers and dimers occluded within AFI channels, and calculated the interatomic distance between protons as the radial distribution function between selected groups of H atoms (Fig. 6). Overall, results show a stronger intermolecular interaction (shorter intermolecular distances) between all protons when they arranged as dimers (Fig. 6A), given by the higher RDF intensity of dimers between 2 and 7 Å (red line). This involves a higher packing efficiency for dimers, as previously observed. Such shorter inter-proton distances for dimers is a consequence of the interaction between aromatic protons composing the dimer (Fig. 6B, red line), as evidenced by the RDF peak between 3 and 6 Å, which is not present when they arrange as monomers (Fig. 6B, blue line). In addition, if we focus on the intermolecular distances between aromatic and the rest of protons (Fig. 6C), we observe a strong H⋯H intermolecular interaction between 2 and 3 Å for dimers which is absent in monomers; this short dimer distance corresponds to the distance between aromatic protons in para position and H3 (from CH-CH3 group) (see Fig. 1-bottom, yellow dashed arrow). These results are a direct consequence of the high packing efficiency when EPH form supramolecular aggregates. Indeed, if we look at the overall intermolecular H⋯H distances (Fig. 6A), a short distance between 2 and 3 Å is found for dimers, while this short distance shifts to ∼4 Å for monomers, which are in good agreement with the distances found by our proton spin diffusion model, explaining the higher 1H spin diffusion rates for EPH aggregates.
4. Conclusions
In this work, we used high-resolution proton spin diffusion MAS NMR combined with DFT calculations to analyse the supramolecular state of organic structure-directing agents confined within the nanopores of microporous materials, which is a direct consequence of the distinct packing efficiency of the guest species as a function of their aggregation state. A series of two-dimensional spin diffusion spectra allowed us to estimate the distances and interactions between the aromatic rings and their first proton neighbours inside the nanopores. This methodology may be very useful to understand the incorporation of organic species in host–guest nanoporous systems, providing important information on how efficiently guest species pack upon confinement.Author contributions
BBM performed the synthesis and general characterization of the materials; ED performed the NMR study; FLA did the fluorescence spectroscopy study, JPP supervised the work, LGH conceptualized and supervised the work, and did the computational work. All authors wrote and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been financed by the Spanish State Research Agency (Agencia Española de Investigación, AEI) through the project PID2019-107968RB-I00. BBM acknowledges the Spanish Ministry of Economy and Competitivity for a predoctoral (BES-2013-064605) contract. Secretaría General Adjunta de Informática-CSIC is acknowledged for running the calculations, and BIOVIA for providing the computational software. We acknowledge the support of the Label of Excellence for the Centre for zeolites and nanoporous materials by the Region of Normandy (CLEAR). We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).Notes and references
- K. Harris and S. J. Thomas, ChemCatChem, 2009, 1, 223–231 CrossRef CAS.
- M. E. Davis, Top. Catal., 2003, 25, 3–7 CrossRef CAS.
- J. Yu and R. Xu, J. Mater. Chem., 2008, 18, 4021–4030 RSC.
- M. E. Davis, ACS Catal., 2018, 8, 10082–10088 CrossRef CAS.
- L. Gómez-Hortigüela and M. A. Camblor, Introduction to the zeolite structure-directing phenomenon by organic species: general aspects, in Insights into the chemistry of organic structure-directing agents in the synthesis of zeolitic materials. Struc. Bond, ed. L. Gómez-Hortigüela, Springer, Cham, 2017, vol. 175 Search PubMed.
- M. Moliner, F. Rey and A. Corma, Angew. Chem., Int. Ed., 2013, 52, 13880–13889 CrossRef CAS PubMed.
- L. Gómez-Hortigüela and B. Bernardo-Maestro, Chiral organic structure-directing agents, in Insights into the chemistry of organic structure-directing agents in the synthesis of zeolitic materials. Struc. Bond, ed. L. Gómez-Hortigüela, Springer, Cham, 2017, vol. 175 Search PubMed.
- S. K. Brand, J. E. Schmidt, M. W. Deem, F. Daeyaert, Y. Ma, O. Terasaki, M. Orazov and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 5101–5106 CrossRef CAS PubMed.
- J. H. Kang, L. B. McCusker, M. W. Deem, C. Baerlocher and M. E. Davis, Chem. Mater., 2021, 33, 1752–1759 CrossRef CAS.
- T. Álvaro-Muñoz, F. López-Arbeloa, J. Pérez-Pariente and L. Gómez-Hortigüela, J. Phys. Chem. C, 2014, 118, 3069–3077 CrossRef.
- B. Bernardo-Maestro, F. López-Arbeloa, J. Pérez-Pariente and L. Gómez-Hortigüela, J. Phys. Chem. C, 2015, 119, 28214–28225 CrossRef CAS.
- B. Bernardo-Maestro, F. López-Arbeloa, J. Pérez-Pariente and L. Gómez-Hortigüela, Microporous Mesoporous Mater., 2017, 254, 211–224 CrossRef CAS.
- B. Bernardo-Maestro, M. D. Roca-Moreno, F. López-Arbeloa, J. Pérez-Pariente and L. Gómez-Hortigüela, Catal. Today, 2016, 277, 9–20 CrossRef CAS.
- B. Bernardo-Maestro, P. Gálvez, D. González, F. López-Arbeloa, J. Pérez-Pariente and L. Gómez-Hortigüela, J. Phys. Chem. C, 2018, 122, 20377–20390 CrossRef CAS.
- B. Bernardo-Maestro, E. Vos, F. López-Arbeloa, J. Pérez-Pariente and L. Gómez-Hortigüela, Microporous Mesoporous Mater., 2017, 239, 432–443 CrossRef CAS.
- C. Paris and M. Moliner, Role of Supramolecular Chemistry During Templating Phenomenon in Zeolite Synthesis, in Insights into the chemistry of organic structure-directing agents in the synthesis of zeolitic materials. Structure and Bonding, ed. L. Gómez-Hortigüela, Springer, Cham, 2017, vol. 175 Search PubMed.
- A. Corma, F. Rey, J. Rius, M. J. Sabater and S. Valencia, Nature, 2004, 431, 287–290 CrossRef CAS PubMed.
- M. Moliner, Top. Catal., 2015, 58, 502–512 CrossRef CAS.
- F. J. Chen, Y. Xu and H. B. Du, Angew. Chem., Int. Ed., 2014, 53, 9592–9596 CrossRef CAS PubMed.
- Z. H. Gao, F. J. Chen, L. Xu, L. Sun, Y. Xu and H. B. Du, Chem. – Eur. J., 2016, 22, 14367–14372 CrossRef CAS PubMed.
- D. R. Liu, Z. H. Gao, W. W. Zi, J. Zhang, H. B. Du and F. J. Chen, Microporous Mesoporous Mater., 2019, 276, 232–238 CrossRef CAS.
- R. Martínez-Franco, A. Cantín, M. Moliner and A. Corma, Chem. Mater., 2014, 26, 4346–4353 CrossRef.
- R. Martínez-Franco, A. Cantín, A. Vidal-Moya, M. Moliner and A. Corma, Chem. Mater., 2015, 27, 2981–2989 CrossRef.
- F. J. Chen, Z. H. Gao, L. L. Liang, J. Zhang and H. B. Du, CrystEngComm, 2016, 18, 2735–2741 RSC.
- W.-W. Zi, Z. Gao, J. Zhang, J.-H. Lv, B.-X. Zhao, Y.-F. Jiang, H.-B. Du and F.-J. Chen, Microporous Mesoporous Mater., 2019, 290, 109654 CrossRef CAS.
- L. Gómez-Hortigüela, F. López-Arbeloa, F. Corà and J. Pérez-Pariente, J. Am. Chem. Soc., 2008, 130, 13274–13284 CrossRef PubMed.
- S. P. Brown, I. Schnell, J. D. Brand, K. Mullen and H. W. Spiess, J. Am. Chem. Soc., 1999, 121, 6712–6718 CrossRef CAS.
- A. R. Hughes and F. Blanc, CrystEngComm, 2021, 23, 2491–2503 RSC.
- S. P. Brown, Solid State Nucl. Magn. Reson., 2012, 41, 1–27 CrossRef CAS PubMed.
- M. H. Levitt, Encycl. Nucl. Magn. Reson., 2007, 9, 165–196 Search PubMed.
- E. Dib, J. Grand, S. Mintova and C. Fernandez, Chem. Mater., 2015, 27, 7577–7579 CrossRef CAS.
- E. Dib, T. Mineva, E. Veron, V. Sarou-Kanian, F. Fayon and B. Alonso, J. Phys. Chem. Lett., 2018, 9, 19–24 CrossRef CAS PubMed.
- J. Li, S. Li, A. Zheng, X. Liu, N. Yu and F. Deng, J. Phys. Chem. C, 2017, 121, 14261–14268 CrossRef CAS.
- S. Clark, M. Segall, C. Pickard, P. Hasnip, M. Probert, K. Refson and M. Payne, Z. Kristallogr., 2005, 220, 567–570 CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, J. Comput. Chem., 2004, 25, 1463–1473 CrossRef CAS PubMed.
- P. Dauber-Osguthorpe, V. A. Roberts, D. J. Osguthorpe, J. Wolff, M. Genest and A. T. Hagler, Proteins: Struct., Funct., Genet., 1988, 4, 31–47 CrossRef CAS PubMed.
- C. J. Pickard and F. Mauri, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 245101 CrossRef.
- M. da S. Machado, J. Pérez-Pariente, E. Sastre, D. Cardoso, M. V. Giotto, J. L. García-Fierro and V. Fornés, J. Catal., 2002, 205, 299–308 CrossRef CAS.
- B. Elena, G. Pintacuda, N. Mifsud and l. Emsley, J. Am. Chem. Soc., 2006, 128, 9555–9560 CrossRef CAS PubMed.
- C. J. Pickard, E. Salager, G. Pintacuda, B. Elena and L. Emsley, J. Am. Chem. Soc., 2007, 129, 8932–8933 CrossRef CAS PubMed.
- E. Salager, R. S. Stein, C. J. Pickard, B. Elena and L. Emsley, Phys. Chem. Chem. Phys., 2009, 11, 2610–2621 RSC.
- E. Salager, G. M. Day, R. S. Stein, C. J. Pickard, B. Elena and L. Emsley, J. Am. Chem. Soc., 2010, 132, 2564–2566 CrossRef CAS PubMed.
- C. Martineau, J. Senker and F. Taulelle, Annu. Rep. NMR Spectrosc., 2014, 82, 1–57 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1, XRD patterns, Fig. S2, diffuse reflectance UV-VIS spectra, Fig. S3, thermogravimetric analyses, Fig. S4, SEM images, Fig. S5, solid state 13C MAS NMR spectra, Fig. S6, solid state 27Al MAS NMR spectra, Fig. S7, solid state 31P MAS NMR spectra, Fig. S8, liquid 1H NMR, Fig. S9, measured peak intensities as a function of spin diffusion mixing time. See DOI: 10.1039/d2dt00497f |
| This journal is © The Royal Society of Chemistry 2022 |