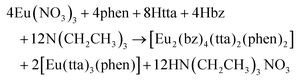Open Access Article
Open Access ArticleHighly luminescent mixed-ligand bimetallic lanthanoid(III) complexes for photovoltaic applications†
Gabriela
Brito-Santos
 a,
Cecilio
Hernández-Rodríguez
a,
Cecilio
Hernández-Rodríguez
 bc,
Beatriz
Gil-Hernández
ac,
Benjamín
González-Díaz
bc,
Beatriz
Gil-Hernández
ac,
Benjamín
González-Díaz
 d,
Inocencio R.
Martín
bc,
Ricardo
Guerrero-Lemus
d,
Inocencio R.
Martín
bc,
Ricardo
Guerrero-Lemus
 bc and
Joaquín
Sanchiz
bc and
Joaquín
Sanchiz
 *ac
*ac
aDepartamento de Química, Facultad de Ciencias, Universidad de La Laguna, Tenerife, 38206, Spain. E-mail: jsanchiz@ull.edu.es
bDepartamento de Física, Facultad de Ciencias, Universidad de La Laguna, Tenerife, 38206, Spain
cInstituto Universitario de Materiales y Nanotecnología, Universidad de La Laguna, Tenerife, 38206, Spain
dDepartamento de Ingeniería Industrial, Escuela Superior de Ingeniería y Tecnología, Universidad de La Laguna, Tenerife, 38206, Spain
First published on 28th January 2022
Abstract
Six new mixed-ligand bimetallic complexes [Eu2(bz)4(tta)2(phen)2] (1), [Gd2(bz)4(tta)2(phen)2] (2), [EuTb(bz)4(tta)2(phen)2] (3), [EuGd(bz)4(tta)2(phen)2] (4), [Eu1,2Gd0,8(bz)4(tta)2(phen)2] (5) and [Eu1,6Gd0,4(bz)4(tta)2(phen)2] (6) have been prepared with the Eu3+, Gd3+ and Tb3+ ions and the benzoate (bz−), 2-thenoyltrifluoroacetonate (tta−) and the 1,10-phenanthroline (phen) ligands. The compounds combine highly efficient antennas to obtain highly luminescent complexes to enhance solar cell efficiency. The benzoate ligand has been chosen to take its advantage as a bridging ligand to end up with bimetallic complexes to study the effect of combining two metal ions in the luminescent molecule. The structure of 1 was obtained by single-crystal X-ray diffraction, and 1–6 were found to be isostructural by powder X-ray diffraction analysis. The photophysical properties were studied by the absorbance and emission spectra and emission lifetimes. The magnetic properties of 2 were studied, and we found intramolecular antiferromagnetic interactions between the Gd3+ ions. We prepared luminescent down-shifting layers (LDSL) with the 1, 3–6 complexes embedded in ethylene–vinyl–acetate and studied their effect in the external quantum efficiency (EQE) and intensity–voltage (I–V) plots of a solar mini-module. We found that LDSL containing the bimetallic complexes 3 and 6 enhance the efficiency of the solar mini-module from 11.26(3)% to 11.76(4)% (+0.52%) and to 11.44(2)% (+0.21%), respectively.
Introduction
Photoluminescence (PL) is one of the most characteristic properties of lanthanoid3+ ions. This phenomenon is particularly intense for Eu3+ and Tb3+ and leads compounds containing those ions to have multiple applications in fields such as LEDs, photovoltaics, temperature and UV sensing or bioimaging, to name a few.1–15 Although the photoluminescence process can be efficient, lanthanoid3+ ions themselves feature low absorption rates, limiting the emission intensity. However, the preparation of suitable lanthanoid ion complexes considerably enhances the photoluminescence intensity by the so-called antenna effect produced by organic sensitizers.16,17 It is commonly accepted that the sensitised luminescence process starts with the excitation of the organic ligand from the singlet ground state to an excited singlet state (S0 → Sn*). At this point, the ligand can participate in inter-system crossing to a lower energy triplet state (ISC). Next, the energy is transferred to an emissive state of the lanthanoid3+ ion, and finally, the lanthanoid3+ centred emission occurs.18–20 The global process is called down-shifting (DS) as it results in an increase in the wavelength of the emitted radiation compared to that absorbed. This phenomenon is of particular interest for photovoltaics (PV) since it simultaneously allows the conversion of inefficient UV photons into more efficient visible ones and protects the photovoltaic device from the UV radiation responsible for the ageing of the photovoltaic modules.2,10,21,22 For these applications, the higher the quantum yield of the overall process, the greater the interest of the compound. The best way to incorporate these compounds in the PV technology consists of their inclusion in the ethylene–vinyl acetate (EVA) or polymethylmethacrylate (PMMA) films that cover the solar modules. For such a purpose, the compounds need to be miscible and processable with those polymers.The final intensity of the emission depends on the efficiency of the different stages: absorption, intersystem crossing (ISC), energy transfer (ET), and radiative deactivation of the lanthanoid ion.16,23–25 Therefore, to have an intense luminescence, we have to prepare complexes with ligands capable of absorbing a wide range of UV wavelengths, favouring the transition to the paramagnetic triplet state of the ligands, enhancing the radiative deactivation respect to the non-radiative processes. For such a purpose, we have prepared new Eu3+, Gd3+ and Tb3+ bimetallic complexes, which contain three different organic ligands (benzoate, bz−; phenanthroline, phen; and 2-thenoyltrifluoroacetonate, tta−), which have never been combined simultaneously but which are known to work efficiently as antennas for Eu3+.17,26–28 The combination of these three ligands gives a broad absorbance from wavelengths ranging from 220 nm to almost 400 nm. Additionally, the characteristics of the chosen ligands minimise the entry of water molecules in the metal ions’ coordination sphere and enhance the processability of the material in PMMA or EVA films, which is of particular interest for photovoltaic modules.14,29,30 We have also taken advantage of the benzoate ligand to work as a bridging ligand, which allowed us to prepare bimetallic complexes,2,31 which in some cases have shown more intense luminescent signal than the corresponding mononuclear analogues.32,33 Also, the benzoate ligand has a high energy triplet state that leads to the population of excited states of Tb3+ that tta− could not populate.31,34 Due to the similar sizes of the lanthanoid ions, isostructural complexes for Eu3+, Gd3+ and Tb3+ are frequently obtained under otherwise the same reaction conditions, which facilitates the synthesis of hetero-bimetallic complexes.35,36
One of the interests of hetero-bimetallic complexes lies in the possibility of combining in the same compound the highly emissive Eu3+ ion with highly paramagnetic ions, such as Gd3+ or Tb3+, which favour the intersystem crossing and reduces the concentration quenching resulting in enhanced luminescent species. It has been reported that the paramagnetic character of the Gd3+ and Tb3+ ions favour the transition from the singlet states of the ligands to their triplet states, minimising ligand fluorescence or vibrational deactivation.16,18 Another exciting aspect of the bimetallic complexes resides in the potential intermetallic energy transfer and potential tuneable emission which may have applications in the field of LEDs, optical sensors, temperature sensing, and bioimaging.8,31,36–40
Thus, as a continuation of our work searching for efficient down-shifters for photovoltaic applications, in this work, we study the synthesis, the structure, the photoluminescence of six new compounds with the general formula [M1M2(bz)4(phen)2(tta)2] where M1 and M2 can be Eu3+, Gd3+, Tb3+ and we study the effect of M1 and M2 on the photoluminescence. Moreover, we have encapsulated a photovoltaic mini-module with the compounds dispersed in an EVA film and studied the increase in the external quantum efficiency (EQE) and the evolution of the intensity–voltage (I–V) curves to quantify the improvement of the energy conversion in the photovoltaic process.
Experimental
Materials
All chemicals and reagents, specifically ethanol, dichloromethane, triethylamine (98%), benzoic acid (Hbz, 99.5%), 2-thenoyltrifluoroacetone (Htta, 99%), 1,10-phenanthroline (phen, 99%), UV transparent ethylene–vinyl–acetate (EVA), Eu(NO3)3·5H2O (99.99%), Gd(NO3)3·6H2O (99.99%), and Tb(NO3)3·5H2O (99.99%), were commercially available and used without further purification unless otherwise stated. All the synthetic procedures were performed under a dinitrogen atmosphere (Scheme 1). | ||
| Scheme 1 Schematic drawing of 2-thenoyltrifluoroacetone (Htta), A; benzoic acid (Hbz), B; and 1,10-phenanthroline (phen), C. | ||
General methods
Elemental analyses (C, H, N, S) and FT-IR (as KBr disks, between 400 cm−1 and 4000 cm−1) were recorded on a FLASH EA 1112 CHNS-O microanalyser and a Thermo NicolletAvatar 360 FT-IR spectrometer, respectively. UV-visible spectra between 220 nm and 800 nm were recorded on a Varian Cary 50 bio UV-Visible Spectrophotometer with samples dissolved in ethanol. Magnetic susceptibility measurements on polycrystalline samples were performed on a Quantum Design MPMS5XL SQUID magnetometer. X-ray powder diffraction patterns were recorded on a PANanalytical X′pert X-ray diffractometer with Cu Kα radiation, 1.54184 Å, at room temperature.Synthesis of the complexes
[Gd2(bz)4(tta)2(phen)2] (2). Yield 53.9 mg (54%). Elemental analysis (%) calcd for C68H44N4Gd2O12F6S2 (1601.76): C, 50.99; H, 2.77; N, 3.50; S, 4.00. Found: C, 49.13; H, 2.42; N, 3.57; S, 5.31. IR (KBr, n/cm−1): 3065(m), 3024(m), 2926(m), 1603(s), 1572(s), 1533(m), 1410(s), 1308(s), 1182(s), 1138(s), 1061(w), 837(m), 783(m), 717(s). UV-vis (ethanol, lmax/nm): 227, 230, 267, 271, 292, 346.
Single-crystal X-ray structure determination
| Empirical formula | C68H44Eu2F6N4O12S2 |
|---|---|
| a R 1 = [∑(||Fo| − |Fc||)/∑|Fo|]. b wR2 = [∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]]1/2 goodness-of-fit S = [∑[w(Fo2 − Fc2)2]/(n − p)]1/2. | |
| M/g mol−1 | 1591.11 |
| Temperature (K) | 293 |
| λ/Å | 1.54184 |
| Crystal system, space group | Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a, b, c(Å) | 10.4570(4), 12.0687(6), 14.0081(7) |
| α, β, γ (°) | 94.967(4), 103.974(4), 111.457(4) |
| V (Å3) | 1566.40(13) |
| Z | 1 |
| D calc/g cm−3 | 1.687 |
| μ/(mm−1) | 15.539 |
| Theta range | 4.012–72.648 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 462, 6027, 5440 462, 6027, 5440 |
| R int | 0.057 |
| R 1 [I > 2σ(I)]a | 0.0487 |
| wR2 [I > 2σ(I)]b | 0.1072 |
GOF on F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
1.003 |
All non-hydrogen atoms in the structure were refined with anisotropic thermal parameters.
The hydrogen atom in α position of 2-thenoyltrifluoroacetonate, H3B, was found in the differences map and it was refined using a DFIX restraint for its C–H distance. Aromatic hydrogen atoms were all positioned geometrically (C–H = 0.93 Å) and refined using a rigid model (AFIX 43) with Uiso(H) = 1.2Ueq(C).
Since the thermal anisotropic displacement parameters indicated prolate ellipsoids, all non-hydrogen atoms of the thiophene ring and benzoate group labelled C were refined with RIGU and SIMU restraints; leading the thermal parameters to behave more properly. In addition, the thiophene ring displays typical substitutional disorder for this group of adjacent sulphur and carbon atoms. This substitutional disorder was modelled with PART commands and DFIX restraints.
Photoluminescence measurements
All the measurements were performed on polycrystalline solid-state samples. Emission measurements were recorded on a Photoluminescence Spectrometer FLS 1000. The samples were excited with a 450 W Xe arc lamp with λexc = 350 nm. The lifetimes were measured with a LeCroy WS 424 Oscilloscope, where an EKSPLA/NT342/3/UVE Optical Parametric Oscillator (OPO) laser was used to excite the sample. The absolute fluorescence quantum yields (PLQY) were calculated using the ‘Indirect Method’ with an FLS1000 fluorimeter (Edinburgh company) and an integration sphere. In this method, the quantum yield is calculated with eqn (1). | (1) |
EQE and I–V measurements of the PV mini-module covered with the Luminescent down-shifting films
For the EQE measurements, UV to IR optical radiation was recorded with the Oriel Merlin Digital Lock-in radiometry system (control unit, preamplifier, and optical chopper 8–1100 Hz) with a sensitivity of 0.5 μV. The typical values measured are in the range of 10 mV. Dual Xenon & Quartz Halogen Source (250–2500 nm) housing a 75 W short-arc xenon lamp and a 100 W quartz halogen lamp with automated selection through an Oriel Cornerstone 260 motorised high resolution 1/4 m monochromator, scanning from 280 nm to 2200 nm was used. The Optical system includes TracQ-Basic software. We have followed the reference cell method50 and the standard recommendations of previous works.3,51 A standard crystalline silicon cell from Rera Solutions BV was used as a reference, from which we have determined the input power. The external quantum efficiency (EQE) was calculated following eqn (2), where Isc is the short circuit current, PL is the power of the incident light, λ is the wavelength, e is the elementary charge, h is the Planck's constant, and c is the speed of light.42 | (2) |
The mini-module employed for the EQE and I–V measurements was fabricated and encapsulated with EVA in the Fraunhofer ISE. It exhibits an efficiency of 11.26(3)%.
I–V curves were measured with a Keithley 2400 Source Meter and an Abet Technologies Solar Simulator that produces one sun output. This station includes I–V measurement software with an interface compatible with Windows 10. The software displays the raw I–V curve and calculates the critical cell performance parameters such as the short circuit current density (Jsc) and the efficiency (η) with a current measurement sensitivity and efficiency measurement resolution of 0.05 μA and 0.02%, respectively.
Results and discussion
Synthesis and X-ray powder pattern diffractograms
The synthesis and characterization of [Eu2(bz)4(tta)2(phen)2] (1), [Gd2(bz)4(tta)2(phen)2] (2), [EuTb(bz)4(tta)2(phen)2] (3), [EuGd(bz)4(tta)2(phen)2] (4), [Eu1,2Gd0,8(bz)4(tta)2(phen)2] (5) and [Eu1,6Gd0,4(bz)4(tta)2(phen)2] (6) is a continuation of our studies in the photovoltaics (PV) applications of [Eu2(bz)6(phen)2] and [Eu(tta)3(phen)].2,3,22,52–54 The excellent luminescent properties of the latter are known for more than 50 years,28 and the former is dinuclear and also very luminescent with the benzoate acting as terminal and bridging ligand. Despite the high luminescence of [Eu2(phen)2(bz)6], its absorbance is very low above 300 nm and, therefore, with limited applications as a down-shifter in PV. On the other hand, the tta− ligand has a high absorbance around 350 nm, which makes [Eu(phen)(tta)3] very active in a spectral region more significant in solar radiation. So, we came up with the idea of preparing a compound that would combine a significant absorbance in the near-UV (we need tta−) with a bimetallic character (we need bz−) to explore the effect in the PV applications of including more than one metal ion in a highly luminescent molecule.With that idea in mind, we tried to obtain [Eu2(bz)4(tta)2(phen)2] (1) with the ratio of reagents corresponding to its stoichiometry. However, we obtained a compound with an elemental analysis corresponding to the formula [Eu2(bz)5(tta)(phen)2] but for which we were not able to obtain a single-crystal suitable for X-ray diffraction. However, we could confirm that its powder diffractogram did not correspond to [Eu(bz)6(phen)2] nor [Eu(tta)3(phen)] and over time, we were able to verify that it did not correspond to 1. Nevertheless, following these other reagent ratios
A crude product containing a mixture of 1 and [Eu(tta)3(phen)] was obtained; both were identified by X-ray powder diffraction patterns (Fig. S2†). [Eu(tta)3(phen)] was removed washing with acetone, and we ended up with 1 in high purity, as can be seen in elemental analysis and powder diffractograms, but at the cost of lowering the yield. Following this procedure, with the appropriate ratio of the corresponding Ln3+ nitrates (Table S1†), compounds 2–6 were isolated. However, the isostructural Tb2 compound was not obtained. Instead, we obtained an unknown crystalline material whose powder diffractogram did not match that of 1 (Fig. S3†). Compounds 1–6 crystallise with a 9-coordination for the lanthanoid ion. We think that the preference of the Tb(III) ion for a coordination number of 8 results in a different structure.55 We will study the properties of this compound as soon as we have its crystal structure.
We obtained single-crystal suitable for X-ray diffraction of 1 by dissolving the product in THF and precipitating it with hexane vapour in a closed flask. This procedure led to a polycrystalline material suitable for powder X-ray diffraction and other measurements for 2–6. By positive matching of the experimental powder diffractograms with that simulated from the single crystal of 1, we could conclude that the single crystal of 1 corresponded to the bulk material of 1 and that all the compounds were isostructural (Fig. S3†).
The compounds [Eu1,2Gd0,8(bz)4(tta)2(phen)2] (5) and [Eu1,6Gd0,4(bz)4(tta)2(phen)2] (6) have fractional numbers for Eu3+ and Gd3+ since they contain homogeneously distributed the two structurally equivalent molecules [Eu2(bz)4(tta)2(phen)2] and [EuGd(bz)4(tta)2(phen)2] in a certain ratio (Table S1†). In principle, we can estimate each molecule's molar fraction with the ratio of the corresponding Ln3+ nitrates used in the synthesis, and with that ratio, we can calculate the coefficients for Gd and Eu. We confirmed this aspect in the magnetic study.
Crystal structure description
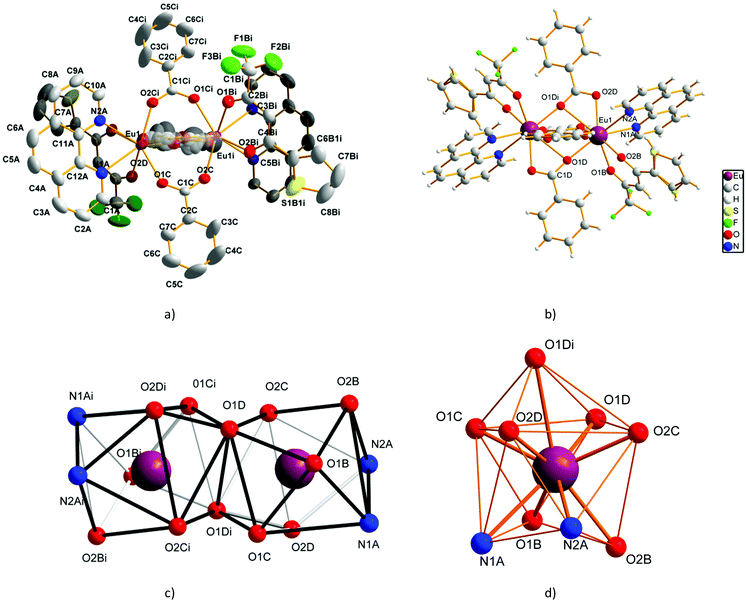
Compounds 1–6 are isostructural and crystallise in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space-group as neutral centrosymmetric dinuclear molecules of [M2(bz)4(tta)2(phen)2] with two 2-thenoyltrifluoroacetonate (tta−), two phenanthroline (phen), and four bridging benzoate (bz−) ligands (Fig. 1a and b and Fig. S5†).
space-group as neutral centrosymmetric dinuclear molecules of [M2(bz)4(tta)2(phen)2] with two 2-thenoyltrifluoroacetonate (tta−), two phenanthroline (phen), and four bridging benzoate (bz−) ligands (Fig. 1a and b and Fig. S5†).
M2 metal-ions can be Eu2, Gd2, EuTb or EuGd. Single-crystals suitable for X-ray diffraction were obtained for the Eu2 compound so that we will discuss compound 1 structure, but the description is valid for the rest with no significant changes in the distances and angles.56 The Eu3+ ions are in nine-coordination with a distorted capped square-antiprism environment (Fig. 1c and d). Two phenanthroline nitrogen atoms (N1A and N2A), two tta− oxygen atoms (O1B and O2B), and five benzoate oxygen atoms (O1C, O1D, O2D, O2C, and O1Di) fill the coordination sphere of the Eu3+ ions with no solvent molecules, [symmetry code: (i) −x, −y + 1, −z + 1]. The bond distances and angles are within the expected values and reported in Table 2 and Table S2.†2,5,31,52,57 The Eu1–O1Di is the longest distance and this atom fills the top position of the caped square-antiprism, Fig. 1d.58
| Symmetry code: (i) −x, −y + 1, −z + 1. | |||
|---|---|---|---|
| Eu1–O1D | 2.341(3) | Eu1–O1B | 2.453(3) |
| Eu1–O2Ci | 2.353(3) | Eu1–N1A | 2.605(4) |
| Eu1–O1C | 2.378(3) | Eu1–N2A | 2.657(4) |
| Eu1–O2B | 2.403(3) | Eu1–O1Di | 2.860(4) |
| Eu1–O2Di | 2.423(3) | Eu1⋯Eu1i | 4.0518(3) |
The tta− and phen act as terminal ligands and appear in trans position to each other. The chelating mode, the distances, and the angles are typical for these ligands. The thiophene ring of the tta− ligand shows disorder in the C6B1 and S1B1 atoms because of the C4B–C5B rotation. C–H⋯π intramolecular interactions take place between thiophene C–H and the phenanthroline ring with a C–H⋯phenanthroline-centroid distance of 2.800(2) Å.
The carboxylate groups of the four benzoate ligands bridge the two Eu3+ atoms. Two of them adopt the symmetric syn–syn mode μ-κO:κO′ (Fig. 1a), whereas the other two appear in a chelating–bridging fashion with a μ-O:κ2O,O′ coordination mode (Fig. 1b). The combination of these four bridges results in an Eu3+⋯Eu3+ intramolecular distance of 4.0518(3) Å, which is shorter than that found in [Eu2(bphen)2(bz)6], 5.5441(1) Å, for which two benzoates act as bridging in the μ-κO:κO′ mode; but longer than that found in [Eu2(phen)2(bz)6], 3.9604(1) Å, with an equivalent bridging mode to that of 1, two μ-κO:κO′ and two μ-O:κ2O,O′ (bphen = 4,7-biphenyl-1,10-phenanthroline).2,52 The Eu–O–Eu and the Eu–O–C angles in the μ-O:κ2O,O′ bridges are designed as θ and φ parameters and are considered of special importance for the description of the magnetic properties (Fig. S6†). These parameters take values of 101.903(2)° and 84.881(2)° for θ and φ, respectively.59
Each dinuclear unit is linked to other six by intermolecular C–H⋯π interactions between benzoate C–H and thiophene rings and between phenanthroline C–H and thiophene rings to afford a two-dimensional supramolecular network (benzoate H⋯thiophene centroid, 2.8875(1) Å; phenanthroline H⋯thiophene centroid 2.9595(1) Å), (Fig. S7†). The minimum Eu3+⋯Eu3+ intermolecular distance is 8.9743(5) Å.
Magnetic properties
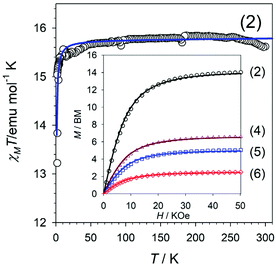
The temperature dependence of the χMT product for compound 2 is shown in Fig. 2, where χM corresponds to the magnetic susceptibility per two Gd3+ ions. The value observed at room temperature is 15.58 emu mol−1 K; somewhat lower than that expected for two magnetically isolated Gd3+ ions [expected value 15.78 emu mol−1 K with g = 2.0 and S = 7/2]. On lowering T, the χMT product slightly increases to reach the expected value and then remains almost constant up to 50 K. Finally continuously decreases to reach a minimum value of 13.21 emu mol−1 K at 2 K. This behaviour is indicative of the existence of weak intramolecular antiferromagnetic interactions between the Gd3+ ions in 2. Gd3+ ions have an 8S7/2 ground state, and therefore, they are very isotropic and significant zero-field-splitting effects are not expected or cannot be unambiguously determined in polycrystalline samples.60,61 Also, we can discard intermolecular interactions since the interaction becomes noticeable just below 50 K and the Gd3+⋯Gd3+ intermolecular distance is above 11 Å.The intramolecular magnetic coupling occurs through the two symmetric syn–syn and the two oxo-carboxylate bridges; the μ-κO:κO′ and the μ-O:κ2O,O′, respectively, Fig. 1a and b. Under that approach, the magnetic data were analysed with the corresponding equation for two interacting spin octets derived from the isotropic spin Hamiltonian Ĥ = −JŜ1Ŝ2.56,62 The calculated curve (Fig. 2 solid dark blue line) fitted well the data and the best-fit parameters were found to be g = 2.00(1), J = −0.021(1) cm−1 and R = 1.14 × 10−4. (R is defined as 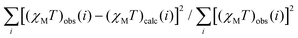 ).
).
Gd3+ dinuclear compounds connected by carboxylate bridges may exhibit different bridging modes, described as A, B and C according to Baggio et al.59,63 Several experimental and theoretical magneto-structural studies have been performed in this kind of compounds, but a clear correlation related to a specific parameter has not been found yet.64 Nevertheless, the most important parameters are the intermetallic Gd3+⋯Gd3+ distances, and the θ and φ parameters (Fig. S6†).59,64 The value obtained for 1 falls within the range previously found in well-characterised Gd3+ dinuclear complexes exhibiting the same kind of bridging mode with similar structural parameters for which values ranging from −0.021 cm−1 to −0.043 cm−1 are reported.59,65,66
The inset of Fig. 2 shows the magnetisation plots as a function of the applied magnetic field for 2, 4–6 at 2 K. At this temperature, the ground state of Gd3+ and Eu3+ ions are the only populated. The Gd3+ ground state 8S7/2 has a spin number S = 7/2, whereas the Eu3+ 7F0 is diamagnetic. Thus the fit of the data to the magnetisation equation can give us the amount of Gd3+ in 2, 4–6. The magnetization equation is given by M = ngSBs(y), where y = gβSH/kT and Bs(y) is the Brillouin function defined by
 | (3) |
| Compound | n expected | n calculated | R | Calculated formula |
|---|---|---|---|---|
a ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)  . .
|
||||
| 2 | 2 | 2 | 0.99 | [Gd2(bz)4(tta)2(phen)2] |
| 4 | 1 | 0.93 | 0.99 | [Eu1.07Gd0.93(bz)4(tta)2(phen)2] |
| 5 | 0.8 | 0.72 | 0.99 | [Eu1.28Gd0.72(bz)4(tta)2(phen)2] |
| 6 | 0.4 | 0.35 | 0.99 | [Eu1.65Gd0.35(bz)4(tta)2(phen)2] |
Photophysical properties
We observe two broad bands centred at 435 nm and 490 nm in the emission spectrum of compound 2, Fig. 4b. The energy of the excited states of the Gd3+ ion is too high to be populated, and the emission spectrum corresponds to the decay of the triplet states of the ligands (phosphorescence). The band at 435 nm corresponds to the deactivation of the triplet state of the benzoate (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1), the other one around 490 nm corresponds indistinguishable to the deactivation of the triplet states of phenanthroline and tta− (20
000 cm−1), the other one around 490 nm corresponds indistinguishable to the deactivation of the triplet states of phenanthroline and tta− (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 cm−1 and 20
790 cm−1 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 cm−1, respectively).33,69 It is noteworthy that in the emission spectra (Fig. 4a), the excitation occurs at 350 nm where only the tta− ligand presents absorption. However, compound 2 shows phosphorescence at wavelengths which do not only correspond to the deactivation of the triplet state of tta− (20
450 cm−1, respectively).33,69 It is noteworthy that in the emission spectra (Fig. 4a), the excitation occurs at 350 nm where only the tta− ligand presents absorption. However, compound 2 shows phosphorescence at wavelengths which do not only correspond to the deactivation of the triplet state of tta− (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 cm−1). This indicates the existence of intersystem crossing (ISC) from the excited singlet states of the tta− (28
450 cm−1). This indicates the existence of intersystem crossing (ISC) from the excited singlet states of the tta− (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 cm−1) not only to the triplet state of tta− but also to those of benzoate and phenanthroline ligands. The activation of the triplet state of the benzoate enables the population of the 5D4 excited state of Tb3+ in 3, since the triplet state of tta− does not have enough energy (20
900 cm−1) not only to the triplet state of tta− but also to those of benzoate and phenanthroline ligands. The activation of the triplet state of the benzoate enables the population of the 5D4 excited state of Tb3+ in 3, since the triplet state of tta− does not have enough energy (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 cm−1) to populate the excited states of Tb3+ (5D4 20
450 cm−1) to populate the excited states of Tb3+ (5D4 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 cm−1).34 It has to be noted that at least 2500 cm−1 of difference are required for an effective ET from the triplet state of the ligand to the Tb3+ to avoid the back-transfer (BT).1,20
430 cm−1).34 It has to be noted that at least 2500 cm−1 of difference are required for an effective ET from the triplet state of the ligand to the Tb3+ to avoid the back-transfer (BT).1,20
In order to investigate a hypothetical energy transfer from Tb3+ (5D4) to Eu3+ (5D1 or 5D0), we measured the excitation spectrum of compound 3 detecting at 612 nm (inset Fig. 4b and Fig. S8†). That spectrum shows a broad band in the range of 225 nm to 450 nm corresponding to the absorption of the bz−, phen and tta− ligands, a peak around 465 nm corresponding to the absorption of Eu3+ (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1, 7F0 → 5D2) and a second transition at 490 nm corresponding to the absorption of Tb3+ (7F6 → 5D4, 20
500 cm−1, 7F0 → 5D2) and a second transition at 490 nm corresponding to the absorption of Tb3+ (7F6 → 5D4, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444 cm−1). This latter band in the excitation spectrum of 3 confirms the Tb3+ → Eu3+ energy transfer.70
444 cm−1). This latter band in the excitation spectrum of 3 confirms the Tb3+ → Eu3+ energy transfer.70
Fig. 5 shows the schematic energy levels diagram of the ground and excited states of the metal ions and ligands and the energy transfers involved in the phosphorescence of the ligands and the emission of the Tb3+ and Eu3+ ions. The activation process in the tta− ligand is shown from the ground singlet state to the excited singlet state, then ISC among the excited singlet state of the tta− to its triplet states, and those of the other ligands occurs followed by the energy transfer (ET) to the Tb3+ and Eu3+ ions, and finally the decay of the excited states of the metal ions to produce the emission.
| Compound | 〈τ〉/ms | PLQY/% | Ref. |
|---|---|---|---|
| a Average lifetime of the 5D4 level for Tb3+ in 3. | |||
| [Eu2(bz)4(tta)2(phen)2] (1) | 0.60 | 63 | This work |
| [EuTb(bz)4(tta)2(phen)2] (3) | 0.75 | 66 | This work |
| 0.0008a | |||
| [EuGd(bz)4(tta)2(phen)2] (4) | 0.65 | 50 | This work |
| [Eu1.2Gd0.8(bz)4(tta)2(phen)2] (5) | 0.72 | 70 | This work |
| [Eu1.6Gd0.4(bz)4(tta)2(phen)2] (6) | 0.74 | 74 | This work |
| [Eu(tta)3(phen)] | 0.98 | 69 | 45 |
| [Eu2(bz)6(phen)2] | 1.02 | 48 | 57 |
| [Eu(tta)3(DBSO)2] | 0.71 | 85 | 68 and 71 |
The values are in the range of 50–74% for 1, 3–6 complexes. All the compounds exhibit higher PLQY than [Eu2(bz)6(phen)2](48%), which means that the inclusion of tta− results in an improvement of the efficiency in the PL process.57 Compared with [Eu(tta)3(phen)](69%), we find somewhat lower, but comparable, values for 3 and 4 that have lower Eu3+ content. However, the high Eu3+ content in 1 results in a low PLQY by concentration quenching. The highest values are found for 5 and 6, which combine the occurrence of the Gd3+ ion and high Eu3+ content. In these compounds, the presence of the Gd3+ ion has a double effect: on the one hand, it decreases the concentration of Eu3+, which results in a decrease in quenching by concentration and on the other hand, the paramagnetic character of Gd3+ favours the ISC in the ligands with respect to the compounds containing only the Eu3+ ion.1 The sum of these two effects contributed by the bimetallic character of the compounds results in an improvement of the PLQY.
In the case of compound 3, the paramagnetic character of Tb3+ leads to higher PLQY values to those of 4 with the same relative Eu3+ content.
The decay curves of the Eu3+ (5D0 → 7F2) transitions are shown in Fig. 6b and Fig. S9.† The curves are nearly exponential, and for all the samples, we calculated the average lifetimes 〈τ〉 of the 5D0 (Eu3+) level with eqn (4), where I(t) is the emission intensity at a time t after pulse excitation at t = 0.71
 | (4) |
The average lifetimes are shown in Table 3 and plotted in Fig. 6a as a function of the Eu3+ content. They take values around 0.77 ms for the samples with low Eu3+ content and decrease to 0.6 ms for the samples with high Eu3+ content. The values are in agreement with those previously reported for similar complexes.18,33 The unusual low value observed in the lifetime of the 5D4 level of Tb3+ in 3 agrees with a fast deactivation via ET to the Eu3+ and/or back transfer to the tta− ligand.34,70
EQE and I–V plots of the PV mini-module covered with the luminescent down-shifting films
The external quantum efficiency (EQE) corresponds to the number of charge carriers collected generated by the solar cell (SC) per incident photon on the device. The measurement of the EQE as a wavelength function shows the SC response and gives the SC characteristics. Studying the so-called intensity–voltage (I–V) curves allows establishing a relationship between the characteristics of the solar cell and its ability to transform solar radiation into electrical energy. The EQE as a function of the wavelength is shown in Fig. 7 for the bare mini-module (m-m), the mini-module encapsulated with pure ethylene–vinyl–acetate (EVA) and covered with a glass (m-m + EVA + glass), and the mini-module encapsulated with the luminescent down-shifting layer (LDSL) containing compounds 1, 3–6 dispersed in EVA and covered with a glass, m-m + EVA(1, 3–6) + glass.The bare and pure EVA encapsulated mini-module is effective in the 375–1150 nm range with a maximum of EQE at 625 nm; this behaviour is typical for multi-crystalline silicon photovoltaic devices. Once the device is covered with films containing 1, 3–6 complexes, an appreciable increase in EQE (ΔEQE) is found in the 300–400 nm spectral range, reaching maximum values in the 9.7% to 16.3% range (Table 5). This increase results from the down-shifting of the ultraviolet radiation in the LDS layer produced by the 1, 3–6 complexes, and it is restricted to this spectral range since the DS process occurs just in this range, as we found in the PL study and absorbance plot, Fig. 3–6.3,72
| Device | ΔJSC (mA cm−2) | ΔJSC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (mA cm−2) a (mA cm−2) |
ΔEQEmax![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
ΔEQEmax![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a,b (%) a,b (%) |
|---|---|---|---|---|
| a With reflector. b At 370 nm. | ||||
| m-m + EVA(3) + glass | 0.15 | 0.38 | 16.3 | 33.8 |
| m-m + EVA(6) + glass | 0.12 | 0.31 | 14.3 | 29.6 |
| m-m + EVA(1) + glass | 0.07 | 0.18 | 9.7 | 20.0 |
| m-m + EVA(4) + glass | 0.09 | 0.23 | 11.4 | 24.0 |
| m-m + EVA(5) + glass | 0.09 | 0.22 | 10.9 | 22.7 |
The higher increase in the EQE is found for the heterobimetallic compounds 3 and 6, 16.3% and 29.6%, respectively, which were found to exhibit high quantum yields in the photoluminescence study. It is also worth noting that EVA(1) shows the lowest activity, which could be explained by the greater effect of quenching by concentration in 1. EVA(4) and EVA(5) show a decrease in activity above 700 nm. This behaviour is attributed to experimental limitations of the assembly of the film on the mini-module (the large number of micro shunts, the irradiation of the mini-modules in a limited area and the position of the effective area of the cell) than to a loss of efficiency in the device due to the encapsulation.73,74 In any case, this decrease has a negative impact in the measured energy conversion, as we discuss later.
Fig. 8 shows the EQE as a function of the wavelength for the same device represented in Fig. 7, but with the device covered with an aluminium reflector that redirects most reflected and re-emitted photons to the SC (Scheme 2). Under these conditions, the EQE values result multiplied by a factor of 2, reaching values in the 20.0% to 33.8% range, indicating that half of the DS photons are not harvested in standard conditions (Table 5). This agrees with the isotropic nature of the emission that projects half of the DS photons out of the SC.75
 | ||
| Fig. 8 EQE plot in the 300–1200 nm spectral range of the mini-module with the different LDS layers covered with an aluminium hemispherical reflector. | ||
To quantify the effect of the LDS layers, we calculated the increase in photo-generated current, ΔJSC, as a function of the wavelength, λ, through eqn (5).76
 | (5) |
To further investigate the impact of LDS layers on the performance of the mini-modules, we measured the I–V curves under standard conditions (irradiance of 1000 W m−2, 25 °C, and AM 1.5), and they are shown in Fig. 9. The plots follow the typical profile with short-circuit current intensity (ISC) in the 2.50–2.75 A range and open-circuit voltage (VOC) of almost 0.58 V.
The characteristic parameters of the solar cell, such as the short circuit current density (Jsc) and the efficiency (η) through the maximum power generated by the solar cell, are shown in Table 6. With the values obtained for the mini-module encapsulated with pure EVA (m-m + EVA + glass), we calculated the gain in JSC (ΔJSC) and efficiency (Δη) for the LDSL EVA(1, 3–6) which are also shown in Table 6.
| Device | J sc (mA cm−2) | ΔJsc (mA cm−2) | η (%) | Δη (%) |
|---|---|---|---|---|
| a Values in parenthesis are standard deviation in the last digit. | ||||
| m-m | 27.14(6)a | 12.14(4) | ||
| m-m + EVA + glass | 25.80(4) | 11.26(3) | ||
| m-m + EVA(3) + glass | 26.69(4) | 0.92(4) | 11.76(4) | 0.52(4) |
| m-m + EVA(6) + glass | 26.04(4) | 0.28(4) | 11.44(2) | 0.21(2) |
| m-m + EVA(5) + glass | 25.40(6) | −0.37(6) | 11.18(1) | −0.06(1) |
| m-m + EVA(1) + glass | 25.53(6) | −0.24(6) | 11.18(3) | −0.08(3) |
| m-m + EVA(4) + glass | 24.79(4) | −0.98(4) | 10.72(2) | −0.52(2) |
We observe a slight decrease in the Jsc and η for the encapsulated m-m + EVA + glass with respect to the bare m-m, which results from the absorbance and reflection of the EVA and the covering glass. Nevertheless, we obtain an increase in efficiency, Δη, of 0.52% and 0.21% with LDS layers containing 3 and 6 in the spectral range of the solar simulator (300–1200 nm). On the other hand, the LDS layers with 1, 4, and 5 show a certain decrease in those parameters because of the decrease in EQE observed above 700 nm for these LDS layers.
Conclusions
We have prepared six new bimetallic mixed-ligand coordination compounds and studied their structure, PL properties and PV applications. The combination of different ligands in the coordination sphere of the Ln3+ ions extends the wavelength range absorption, enhancing the excitation of the complexes and resulting in high emission quantum yields. We have observed that bridging ligands such as benzoate may lead to hetero-dinuclear complexes. The magnetic study shows an intermetallic interaction through the carboxylate bridges and confirms the composition of the bimetallic compounds. The benzoate ligand inclusion increases the energy of the lower-energy triplet state by ISC from the singlet tta− state to the benzoate triplet, enabling the population of Tb3+ excited states that would not be possible without this ligand. Also, the photoluminescence study shows that the bi-metallic 3, 5 and 6 compounds containing the paramagnetic Tb3+ and Gd3+ ions exhibit higher PLQY than the homometallic [Eu2(bz)4(tta)2(phen)2]; which indicates that the hetero-bimetallic character improves the complexes PL response.Combining the bridging benzoate with phen and tta− ligands results in molecular species dispersible in EVA polymeric films. The films can be encapsulated on a photovoltaic multi-crystalline silicon mini-module, showing UV radiation absorption and an increase in the efficiency by the down-shifting process for all the compounds in the range 300–400 nm and for compounds 3 and 6 in the spectral range 300–1200 nm.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is part of the project I+D+I RTI2018-095563-B-I00 funded by MCIN/AEI/10.13039/501100011033/FEDER “Una manera de hacer Europa”. Photoluminescence study and measurements were financed by projects I+D+I PID2019-106383GB-C44 and PID2019-107335RA-I00 funded by MCIN/AEI/10.13039/501100011033/FEDER “Una manera de hacer Europa”. GBS thanks predoctoral scholarship PRE2019-087522 funded by MCIN/AEI/10.13039/501100011033 y FSE “El FSE invierte en tu futuro”.References
- K. Binnemans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- T. Monzón-Hierro, J. Sanchiz, S. González-Pérez, B. González-Díaz, S. Holinski, D. Borchert, C. Hernández-Rodríguez and R. Guerrero-Lemus, Sol. Energy Mater. Sol. Cells, 2015, 136, 187–192 CrossRef.
- R. Guerrero-Lemus, J. Sanchiz, M. Sierra, I. R. Martín, C. Hernández-Rodríguez and D. Borchert, Sol. Energy Mater. Sol. Cells, 2018, 185, 312–317 CrossRef CAS.
- T. Fix, A. Nonat, D. Imbert, S. Di Pietro, M. Mazzanti, A. Slaoui and L. J. Charbonnière, Prog. Photovoltaics: Res. Appl., 2016, 24, 1251–1260 CrossRef CAS.
- F. M. Cabral, D. A. Gálico, I. O. Mazali and F. A. Sigoli, Inorg. Chem. Commun., 2018, 98, 29–33 CrossRef CAS.
- T. Gorai, W. Schmitt and T. Gunnlaugsson, Dalton Trans., 2021, 50, 770–784 RSC.
- R. Gao, M. S. Kodaimati and D. Yan, Chem. Soc. Rev., 2021, 50, 5564–5589 RSC.
- S. Wang, J. Jiang, Y. Lu, J. Liu, X. Han, D. Zhao and C. Li, J. Lumin., 2020, 226, 117418 CrossRef CAS.
- T. Y. Popelensky and V. V. Utochnikova, Dalton Trans., 2020, 49, 12156–12160 RSC.
- D. Yang, H. Liang, Y. Liu, M. Hou, L. Kan, Y. Yang and Z. Zang, Dalton Trans., 2020, 49, 4725–4731 RSC.
- C. M. B. Leite Silva, A. G. Bispo-Jr, S. A. M. Lima and A. M. Pires, Opt. Mater., 2019, 96, 109323 CrossRef CAS.
- S. F. H. Correia, A. R. N. Bastos, L. Fu, L. D. Carlos, P. S. André and R. A. S. Ferreira, Opto-Electron. adv., 2019, 2, 19000601–19000608 Search PubMed.
- A. Gavriluta, T. Fix, A. Nonat, A. Slaoui, J.-F. Guillemoles and L. J. Charbonnière, Eur. J. Inorg. Chem., 2017, 5318–5326 CrossRef CAS.
- M. Li, Y. Zhou, Y. Yao, T. Gao, P. Yan and H. Li, Dalton Trans., 2021, 50, 9914–9922 RSC.
- A. Topor, D. Avram, R. Dascalu, C. Maxim, C. Tiseanu and M. Andruh, Dalton Trans., 2021, 50, 9881–9890 RSC.
- Y. Kitagawa, F. Suzue, T. Nakanishi, K. Fushimi and Y. Hasegawa, Dalton Trans., 2018, 47, 7327–7332 RSC.
- A. I. S. Silva, N. B. D. Lima, A. M. Simas and S. M. C. Goncalves, ACS Omega, 2017, 2, 6786–6794 CrossRef CAS PubMed.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS.
- Y. Ma and Y. Wang, Coord. Chem. Rev., 2010, 254, 972–990 CrossRef CAS.
- Y. Hasegawa, Y. Kitagawa and T. Nakanishi, NPG Asia Mater., 2018, 10, 52–70 CrossRef CAS.
- E. Klampaftis, D. Ross, K. R. McIntosh and B. S. Richards, Sol. Energy Mater. Sol. Cells, 2009, 93, 1182–1194 CrossRef CAS.
- B. González-Díaz, M. H. Saw, C. Hernández-Rodríguez, J. Sanchiz, Y. S. Khoo and R. Guerrero-Lemus, Mater. Sci. Eng., B, 2020, 261, 114763 CrossRef.
- L. V. Meyer, F. Schonfeld and K. Muller-Buschbaum, Chem. Commun., 2014, 50, 8093–8108 RSC.
- M. Latva, H. Takalo, V.-M. Mukkala, C. Matachescu, J. C. Rodríguez-Ubis and J. Kankare, J. Lumin., 1997, 75, 149–169 CrossRef CAS.
- X. P. Yang, B. S. Kang, W. K. Wong, C. Y. Su and H. Q. Liu, Inorg. Chem., 2003, 42, 169–179 CrossRef CAS PubMed.
- L. Abad Galan, B. L. Reid, S. Stagni, A. N. Sobolev, B. W. Skelton, E. G. Moore, G. S. Hanan, E. Zysman-Colman, M. I. Ogden and M. Massi, Dalton Trans., 2018, 47, 7956–7964 RSC.
- V. I. Tsaryuk and K. P. Zhuravlev, J. Lumin., 2021, 237, 118159 CrossRef CAS.
- L. R. Melby, N. J. Rose, E. Abramson and J. C. Caris, J. Am. Chem. Soc., 1964, 86, 5117–5125 CrossRef CAS.
- W. Li, P. Yan, G. Hou, H. Li and G. Li, Dalton Trans., 2013, 42, 11537–11547 RSC.
- O. Moudam, B. C. Rowan, M. Alamiry, P. Richardson, B. S. Richards, A. C. Jones and N. Robertson, Chem. Commun., 2009, 6649–6651 RSC.
- Z. Abbas, P. Singh, S. Dasari, S. Sivakumar and A. K. Patra, New J. Chem., 2020, 44, 15685–15697 RSC.
- J. Shi, Y. Hou, W. Chu, X. Shi, H. Gu, B. Wang and Z. Sun, Inorg. Chem., 2013, 52, 5013–5022 CrossRef CAS PubMed.
- H. F. Li, P. F. Yan, P. Chen, Y. Wang, H. Xu and G. M. Li, Dalton Trans., 2012, 41, 900–907 RSC.
- V. V. Khistiaeva, A. S. Melnikov, S. O. Slavova, V. V. Sizov, G. L. Starova, I. O. Koshevoy and E. V. Grachova, Inorg. Chem. Front., 2018, 5, 3015–3027 RSC.
- M. Fairley, M. M. V. S. Livera, W. Chen, J. H. S. K. Monteiro, A. Schmitt, A. de Bettencourt-Dias, S. Roberts and Z. Zheng, J. Rare Earths, 2021, 39, 487–494 CrossRef CAS.
- P. A. Demakov, A. A. Ryadun, P. V. Dorovatovskii, V. A. Lazarenko, D. G. Samsonenko, K. A. Brylev, V. P. Fedin and D. N. Dybtsev, Dalton Trans., 2021, 50, 11899–11908 RSC.
- S. Xing and C. Janiak, Chem. Commun., 2020, 56, 12290–12306 RSC.
- F. Saraci, V. Quezada-Novoa, P. R. Donnarumma and A. J. Howarth, Chem. Soc. Rev., 2020, 49, 7949–7977 RSC.
- W. Gao, H. Wei, C. L. Wang, J. P. Liu and X. M. Zhang, Dalton Trans., 2021, 50, 11619–11630 RSC.
- K. M. Kuznetsov, M. I. Kozlov, A. N. Aslandukov, A. A. Vashchenko, A. V. Medved'ko, E. V. Latipov, A. S. Goloveshkin, D. M. Tsymbarenko and V. V. Utochnikova, Dalton Trans., 2021, 50, 9685–9689 RSC.
- Angilent Technologies, XCalibur CCD System, CRISALISPRO Software System, Version 1.171.36.24, 2012 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- C. B. Hubschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef PubMed.
- K. Brandenburg and H. Putz, Diamond (Version 3.2), Crystal and Molecular Structure Visualization, 2009 Search PubMed.
- F. R. G. Silva, J. F. S. Menezes, G. B. Rocha, S. Alves, H. F. Brito, R. L. Longo and O. L. Malta, J. Alloys Compd., 2000, 303–304, 364–370 CrossRef.
- U. Blieske and G. Stollwerck, in Semicond. Semimet, ed. G. P. Willeke and E. R. Weber, Elsevier, 2013, vol. 89, pp. 199–258 Search PubMed.
- S. K. Gaddam, R. Pothu and R. Boddula, J. Materiomics, 2021, 7, 920–928 CrossRef.
- R. Satpathy and V. Pamuru, Chapter 5 - Manufacturing of crystalline silicon solar PV modules, Academic Press, 2021 Search PubMed.
- M. Spiegel, Sol. Energy Mater. Sol. Cells, 1998, 55, 331–340 CrossRef CAS.
- C. H. Seaman, Sol. Energy, 1982, 29, 291–298 CrossRef CAS.
- R. Guerrero-Lemus, J. Sanchiz, M. Sierra-Ramos, I. R. Martín, C. Hernández-Rodríguez and D. Borchert, Sens. Actuators, A, 2018, 271, 60–65 CrossRef CAS.
- S. González-Pérez, J. Sanchiz, V. D. Rodríguez, D. Cañadillas-Ramallo, J. González-Platas, D. Borchert, B. González-Díaz, C. Hernández-Rodríguez and R. Guerrero-Lemus, J. Lumin., 2018, 201, 148–155 CrossRef.
- B. González-Díaz, M. Sierra-Ramos, J. Sanchiz and R. Guerrero-Lemus, Sens. Actuators, A, 2018, 276, 312–319 CrossRef.
- S. González-Pérez, J. Sanchiz, B. González-Díaz, S. Holinski, D. Borchert, C. Hernández-Rodríguez and R. Guerrero-Lemus, Surf. Coat. Technol., 2015, 271, 106–111 CrossRef.
- J. Cepeda, S. Perez-Yanez, G. Beobide, O. Castillo, J. A. Garcia, M. Lanchas and A. Luque, Dalton Trans., 2015, 44, 6972–6986 RSC.
- A. W.-H. Lam, W.-T. Wong, S. Gao, G. Wen and X.-X. Zhang, Eur. J. Inorg. Chem., 2003, 149–163 CrossRef CAS.
- O. T. Alexander, R. E. Kroon, A. Brink and H. G. Visser, Dalton Trans., 2019, 48, 16074–16082 RSC.
- H. Bußkamp, G. B. Deacon, M. Hilder, P. C. Junk, U. H. Kynast, W. W. Lee and D. R. Turner, CrystEngComm, 2007, 9, 394–411 RSC.
- L. Canadillas-Delgado, O. Fabelo, J. Pasan, F. S. Delgado, F. Lloret, M. Julve and C. Ruiz-Perez, Dalton Trans., 2010, 39, 7286–7293 RSC.
- R. Boča, Coord. Chem. Rev., 2004, 248, 757–815 CrossRef.
- J. W. de Oliveira Maciel, M. A. Lemes, A. K. Valdo, R. Rabelo, F. T. Martins, L. J. Queiroz Maia, R. C. de Santana, F. Lloret, M. Julve and D. Cangussu, Inorg. Chem., 2021, 60, 6176–6190 CrossRef CAS PubMed.
- O. Kahn, Molecular Magnetism, VCH, New York, 1993 Search PubMed.
- R. Baggio, R. Calvo, M. T. Garland, O. Pena, M. Perec and A. Rizzi, Inorg. Chem., 2005, 44, 8979–8987 CrossRef CAS PubMed.
- O. Roubeau, G. Lorusso, S. J. Teat and M. Evangelisti, Dalton Trans., 2014, 43, 11502–11509 RSC.
- S. C. Manna, E. Zangrando, J. Ribas and N. Ray Chaudhuri, Polyhedron, 2006, 25, 1779–1786 CrossRef CAS.
- A. Rohde and W. Urland, Dalton Trans., 2006, 2974–2978 RSC.
- I. Georgieva, N. Trendafilova, T. Zahariev, N. Danchova and S. Gutzov, J. Lumin., 2018, 202, 192–205 CrossRef CAS.
- O. L. Malta, H. F. Brito, J. F. S. Menezes, f. R. G. E. Silva, S. Alves, F. S. Farias and A. V. M. de Andrade, J. Lumin., 1997, 75, 255–268 CrossRef CAS.
- W. R. Dawson, J. L. Kropp and M. W. Windsor, J. Chem. Phys., 1966, 45, 2410–2418 CrossRef CAS.
- A. N. Carneiro Neto, R. T. Moura, A. Shyichuk, V. Paterlini, F. Piccinelli, M. Bettinelli and O. L. Malta, J. Phys. Chem. C, 2020, 124, 10105–10116 CrossRef CAS.
- S. González-Pérez, I. R. Martín, F. Lahoz, P. Haro-González and J. Herreros, Appl. Phys. A, 2008, 93, 983–986 CrossRef.
- R. Rothemund, Sol. Energy Mater. Sol. Cells, 2014, 120, 616–621 CrossRef CAS.
- R. Scheer and H.-W. Schock, Chalcogenide Photovoltaics: Physics, Technologies, and Thin Film Devices, Wiley-VCH Verlag GmbH & Co. KGaA, 2011 Search PubMed.
- F. Fertig, M. Padilla, O. Breitenstein, H. Höffler, I. Geisemeyer, M. C. Schubert and S. Rein, Energy Procedia, 2015, 77, 43–56 CrossRef CAS.
- L. Li, Opt. Lasers Eng., 2000, 34, 231–253 CrossRef.
- J. Liu, K. Wang, W. Zheng, W. Huang, C.-H. Li and X.-Z. You, Prog. Photovoltaics: Res. Appl., 2013, 21, 668–675 CAS.
- ASTM G173-03(2020), Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface, ASTM International, West Conshohocken, PA, 2020, http://www.astm.org, DOI:10.1520/G0173-03R20.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD patterns, additional figures and tables. CCDC 2110403 for complex 1. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1dt04248c |
| This journal is © The Royal Society of Chemistry 2022 |