Synthesis, structure and aromaticity of metallapyridinium complexes†
Abstract
The first rhena-analogues of pyridinium (cyclopropametalla-2-isoquinolinium complexes) are obtained from o-ethynyl benzonitriles. Structural analysis and DFT calculations confirm their aromatic nature. Compared to rhenapyrylium, rhenapyridinium has a slightly stronger Hückel π-aromaticity, while a chlorine substituent on the rhenapyridinium ring decreases its aromaticity, which is revealed by NICS, EDDB, MCI and ΔBV(ELFπ) analysis.
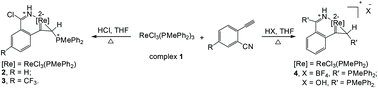


 Please wait while we load your content...
Please wait while we load your content...