Open Access Article
Open Access ArticleFrom ethene to propene (ETP) on tailored silica–alumina supports with isolated Ni(II) sites: uncovering the importance of surface nickel aluminate sites and the carbon-pool mechanism†
Zixuan
Chen
 a,
Scott R.
Docherty
a,
Scott R.
Docherty
 b,
Pierre
Florian
b,
Pierre
Florian
 c,
Agnieszka
Kierzkowska
c,
Agnieszka
Kierzkowska
 a,
Ilia B.
Moroz
a,
Ilia B.
Moroz
 b,
Paula M.
Abdala
b,
Paula M.
Abdala
 a,
Christophe
Copéret
a,
Christophe
Copéret
 *b,
Christoph R.
Müller
*b,
Christoph R.
Müller
 *a and
Alexey
Fedorov
*a and
Alexey
Fedorov
 *a
*a
aLaboratory of Energy Science and Engineering, ETH Zürich, 8092 Zürich, Switzerland. E-mail: muelchri@ethz.ch; fedoroal@ethz.ch
bDepartment of Chemistry and Applied Biosciences, ETH Zürich, 8093 Zürich, Switzerland. E-mail: ccoperet@ethz.ch
cCNRS, CEMHTI UPR3079, University of Orléans, F-45071 Orléans, France
First published on 31st August 2022
Abstract
Catalysts with well-defined isolated Ni(II) surface sites have been prepared on three silica-based supports. The outer shells of the support were comprised either of an amorphous aluminosilicate or amorphous alumina (AlOx) layer – associated with a high and low density of strong Brønsted acid sites (BAS), respectively. When tested for ethene-to-propene conversion, Ni catalysts with a higher density of strong BAS demonstrate a higher initial activity and productivity to propene. On all three catalysts, the propene productivity correlates closely with the concentration of C8 aromatics, suggesting that propene may form via a carbon-pool mechanism. While all three catalysts deactivate with time on stream, the deactivation of catalysts with Ni(II) sites on AlOx, i.e., containing surface Ni aluminate sites, is shown to be reversible by calcination (coke removal), in contrast to the deactivation of surface Ni silicate or aluminosilicate sites, which deactivate irreversibly by forming Ni nanoparticles.
Introduction
The development of processes for the on-purpose production of propene has been spurred in recent decades by the constantly growing demand for this important platform chemical.1,2 Such processes include, for instance, propane dehydrogenation3,4 or methanol-to-propene conversion.5–7 An appealing alternative route is the direct conversion of ethene to propene, i.e. the ETP reaction, which to date is still underdeveloped despite its advantages (mild reaction conditions and potentially atom economy).8 ETP catalysts based on zeolites and Al2O3-supported tungsten hydride have been reported.9–11 Ni-based ETP catalysts have also been developed, such as Ni ions on mesoporous silica, i.e. Ni-MCM-41,12–17 or on the Al-doped mesoporous support, Ni–Al-MCM-41.18 The presence of Al ions induced strong Brønsted acidity of the support and was found to increase the catalytic activity.18 Subsequently, ETP activity has been demonstrated for Ni-based catalysts on a non-mesoporous support, i.e., Al-doped silica,19 and the formation of propene starting from ethanol has been reported as well.20,21 Both isolated sites of Ni(II) and strong Brønsted acidity, arising from the introduction of Al, are thought to be essential for the ETP reaction to proceed.22 Despite those advances and mechanistic insights,23 a detailed understanding of how strong Brønsted acidity influences the catalytic performance of Ni(II) sites in the ETP reaction is still lacking.Atomic layer deposition (ALD) is a versatile approach to prepare supports with well-controlled surface acidity properties.24,25 Here, we utilize ALD of trimethylaluminum on partially dehydroxylated silica to prepare tailored non-mesoporous silica–alumina supports with a controlled density of strong Brønsted acid sites (BAS).26 We then employ the surface organometallic chemistry (SOMC) approach to obtain isolated Ni(II) sites on those supports.22,27 We prepare three catalysts, study their ETP activity and productivity to propene and correlate it to the abundance of strong Brønsted acidity, which scales from high to medium and low depending on the number of ALD cycles (1, 5 and 10, respectively).26 A detailed product analysis identifies that along with C1–C4 hydrocarbons, which are typical products of the Ni-based ETP reaction, a number of aromatic products form as well, including benzene, toluene, xylenes and ethylbenzene (denoted BTXE). We find that the formation rate of propene correlates closely with the formation rate of C8 aromatics, in particular xylenes. This observation is consistent with the cracking of ethene oligomers, produced by the Ni(II) sites, on cationic C8 species (such as xylenes protonated by the strong BAS). In other words, a carbon-pool mechanism is likely responsible for the propene production on Ni-based ETP catalysts containing strong Brønsted acidity; such a pathway is reminiscent of that involved in zeolite-catalyzed ETP conversion,28,29 and also in the methanol-to-olefins process.30,31
Importantly, we also uncover that the use of supports that contain an amorphous alumina (AlOx) overlayer yields catalysts that avoid the formation of nickel nanoparticles (NPs) from isolated Ni(II) sites with time on stream (TOS); the latter is a typical deactivation pathway of Ni-based ETP catalysts. The formation of surface nickel aluminate sites on the AlOx overlayer is proposed to be responsible for the increased stability against deactivation by Ni NPs formation, which likely takes place by the reduction of Ni(II) sites to metallic Ni followed by sintering. In contrast, isolated nickel silicate or aluminosilicate sites form Ni NPs with TOS and therefore do not regenerate fully during coke removal via calcination, while the catalysts with isolated nickel aluminate sites regenerate fully by calcination. Therefore, a combination of strong Brønsted acidity and isolated nickel aluminate sites is proposed to be essential features of active Ni-based ETP catalysts amenable to complete regeneration, while propene is proposed to form, at least in part, via a carbon-pool mechanism.
Results
ALD-derived silica–alumina supports with a controlled abundance of strong Brønsted acid sites were prepared using 1, 5 or 10 ALD cycles of trimethylaluminum onto silica dehydroxylated at 500 °C, as described by us previously.26 This methodology yields supports with a high, medium and low abundance of strong Brønsted acidity. Depending on the number of cycles, the respective materials are denoted Al1-SiO2-500, Al5-SiO2-500 and Al10-SiO2-500 and their surface area ranges between 280 and 160 m2 g−1. According to our previous detailed solid state NMR studies, the ALD-deposited shell (with a silica core) in Al1-SiO2-500 is an amorphous aluminosilicate (ASA), which contains abundant strong BAS.26 In contrast, Al5-SiO2-500 and Al10-SiO2-500 contain layers of AlOx grown on top of the ASA shell (Fig. 1).26 With respect to Al1-SiO2-500, the amorphous alumina shell in Al5-SiO2-500 features a decreased abundance of strong BAS; in Al10-SiO2-500 the abundance of strong BAS is reduced further. Using these supports, three ETP catalysts containing isolated Ni(II) sites were prepared by the SOMC approach.27 This approach relies on a selective reaction between surface sites of a dehydroxylated support (such as isolated silanol or aluminol OH groups) and a molecular complex, viz. a grafting reaction, which in our case is a siloxide ligand exchange reaction between [Ni(OSi(OtBu)3)2]2 and the surface hydroxyl groups of the Al1,5,10-SiO2-500 supports dehydroxylated at 500 °C (Fig. 1).22 Following the grafting reaction, the materials are calcined at 400 °C (see ESI† for details) similarly to our previous report,22 and characterized avoiding exposure to air. Henceforth, the three catalysts prepared are denoted Ni-Al1-, Ni-Al5-, Ni-Al10-SiO2-500 and contain, according to elemental analysis, 0.73%, 1.03%, 1.09 wt% Ni, respectively.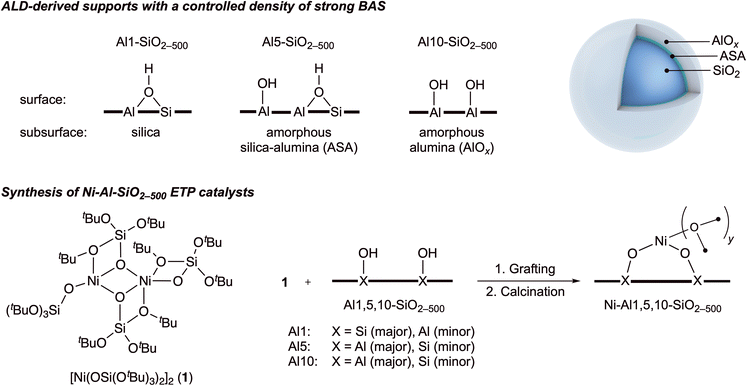 | ||
| Fig. 1 The ALD-SOMC approach to yield catalysts with isolated Ni(II) sites on the ALD-derived alumina–silica supports with a controlled density of strong Brønsted acid sites. The notations AlOx and ASA refer to amorphous alumina and amorphous silica–alumina (an aluminosilicate layer), respectively. The structure of the calcined Ni-Al-SiO2-500 catalysts is shown with X = Al and Si assuming a mono-grafting of [Ni(OSi(OtBu)3)2]2 on Al1-, Al5- and Al10-SiO2-500 supports, and that the calcination of the mono-grafted species may, at least partially, proceed without breaking the Ni–O–Si linkage. See Table 1 for the possible range of values of variable y. | ||
A detailed characterization of the prepared catalysts is reported in the ESI.† In brief, the application of the combined ALD-SOMC approach yields materials that contain isolated Ni(II) sites supported on Al1,5,10-SiO2-500. X-ray absorption near-edge structure (XANES) data suggests a Ni(II) oxidation state in all three catalysts (Fig. S8†). The results of the extended X-ray absorption fine structure (EXAFS) analysis of all three materials reveal the presence of Ni–O and Ni–Al(Si) coordination spheres and are indicative of the presence of isolated Ni(II) sites. The EXAFS data were fitted using Ni–O and Ni–Al/Si paths and the fitting parameters are summarized in Table 1.32 All three Ni-Al1,5,10-SiO2-500 catalysts exhibit two similar coordination spheres in EXAFS. The first coordination sphere is represented by a single Ni–O path while the second coordination sphere is fitted with two Ni–Al(Si) paths at two different interatomic distances. As discussed above, Al1-SiO2-500 support is different from Al5- and Al10-SiO2-500 supports in that Al1-SiO2-500 has an amorphous aluminosilicate (ASA) shell while Al5- and Al10-SiO2-500 supports contain an amorphous alumina (AlOx) shell. Therefore, it is challenging to distinguish between Ni–Si and Ni–Al paths in Ni-Al1-SiO2-500 as the atomic number of Si and Al are close.33,34 It is likely that both Ni–Si and Ni–Al paths are present in Ni-Al1-SiO2-500 while Ni–Al paths dominate the second sphere in Ni-Al5-SiO2-500 and Ni-Al10-SiO2-500 (Fig. S9–S11†). The presence of a Ni–Ni second coordination sphere can be ruled out (see ESI† for additional discussion), hence excluding the possibility of the formation of NiO crystallites (Tables S1 and S2, Fig. S12†). In line with the EXAFS analysis, the characteristic feature of NiO at ca. 8370 eV is absent in the XANES spectra of all three Ni-Al1,5,10-SiO2-500 catalysts. In Ni-Al1-SiO2-500, the Ni sites are likely a combination of both surface nickel silicate and nickel aluminate sites, while in Ni-Al5-SiO2-500 and Ni-Al10-SiO2-500 these are predominantly nickel aluminate sites.
| Material | Neighbour | CN | r (Å) | σ 2 (Å2) | E 0 (eV) | R-Factor |
|---|---|---|---|---|---|---|
| All samples were measured at ambient temperature. S02 was fixed to 0.847 obtained by fitting a Ni foil reference. CN stands for the coordination number. Debye–Waller factor σ2 were constrained to the same value for all paths in each sample. | ||||||
| Ni-Al1-SiO2-500 | O | 4.8(4) | 2.00(3) | 0.009(1) | −4(1) | 0.002 |
| Si(Al) | 1.5(3) | 2.78(8) | 0.009(1) | |||
| Al(Si) | 2.1(4) | 3.22(7) | 0.009(1) | |||
| Ni-Al5-SiO2-500 | O | 4.9(7) | 1.98(6) | 0.009(1) | −8(2) | 0.005 |
| Al(Si) | 1.1(5) | 2.77(9) | 0.009(1) | |||
| Al | 2.1(6) | 3.19(9) | 0.009(1) | |||
| Ni-Al10-SiO2-500 | O | 4.4(4) | 1.99(4) | 0.008(1) | −5(1) | 0.002 |
| Al(Si) | 1.0(3) | 2.76(9) | 0.008(1) | |||
| Al | 1.7(4) | 3.22(7) | 0.008(1) | |||
The 27Al nuclear magnetic resonance (NMR) spectra of Ni-Al1-, Ni-Al5-, Ni-Al10-SiO2-500 display features similar to those of Al1-, Al5-, and Al10-SiO2-500 reported by us previously,26i.e., the spectra contain three overlapping peaks due to four-, five- and six-fold coordinated Al sites (Fig. S36†). The chemical shifts are found to increase with increasing number of ALD cycles, explained by more abundant Al–O–Al linkages (in preference to Al–O–Si linkages). Compared to the spectra of Al1-, Al5-, and Al10-SiO2-500, there is a significant increase of the line width in Ni-Al1-, Ni-Al5- and Ni-Al10-SiO2-500 due to a larger distribution of the isotropic chemical shift values Δδiso (Fig. S36, Table S4†). This points at an increased chemical disorder in the Ni catalysts, likely caused by the SOMC deposition and calcination steps.
Note that a paramagnetic Ni(II) site in our catalysts “bleaches” nearby Al sites, making them invisible in the 27Al NMR spectra. The normalized (with respect to the weight and the number of scans), integrated area of the NMR peaks is found to be 0.95, 4.58 and 9.77 a.u. for Ni-Al1-, Ni-Al5- and Ni-Al10-SiO2-500 respectively, which corresponds to a relative ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8
4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.3 of aluminum atoms in the samples detected by NMR. This ratio is very close to the number of ALD cycles used (i.e., 1, 5 and 10 cycles). In other words, the spatial distribution of Ni and the effect of paramagnetic bleaching is homogeneous for all three samples and no Ni clustering is evidenced by NMR, in agreement with the EXAFS data. Next, we fitted the 27Al NMR spectra of Ni-Al5- and Ni-Al10-SiO2-500 using the spectrum of Ni-Al1-SiO2-500 as a model of the SiO2–AlOx interface. The bleaching effect of Ni(II) explains the lower fitted fraction of the Ni-Al1-SiO2-500 component in Ni-Al5-SiO2-500 relative to Al5-SiO2-500 (21% and 51%, respectively, Table S4;† the SiO2–AlOx interface has been modelled in Al5-SiO2-500 and Al10-SiO2-500 using the spectrum of Al1-SiO2-500).26 The same effect is observed in Ni-Al10-SiO2-500 relative to Al10-SiO2-500 (30% and 37%, respectively), although it is notably less pronounced. Therefore, the differences in the fitting results of the Ni-Al1-SiO2-500 component suggest that in Ni-Al5-SiO2-500 a large fraction of the Ni(II) sites are located in the vicinity of the strong BAS of the SiO2–AlOx interface, yet this is not the case in Ni-Al10-SiO2-500.
10.3 of aluminum atoms in the samples detected by NMR. This ratio is very close to the number of ALD cycles used (i.e., 1, 5 and 10 cycles). In other words, the spatial distribution of Ni and the effect of paramagnetic bleaching is homogeneous for all three samples and no Ni clustering is evidenced by NMR, in agreement with the EXAFS data. Next, we fitted the 27Al NMR spectra of Ni-Al5- and Ni-Al10-SiO2-500 using the spectrum of Ni-Al1-SiO2-500 as a model of the SiO2–AlOx interface. The bleaching effect of Ni(II) explains the lower fitted fraction of the Ni-Al1-SiO2-500 component in Ni-Al5-SiO2-500 relative to Al5-SiO2-500 (21% and 51%, respectively, Table S4;† the SiO2–AlOx interface has been modelled in Al5-SiO2-500 and Al10-SiO2-500 using the spectrum of Al1-SiO2-500).26 The same effect is observed in Ni-Al10-SiO2-500 relative to Al10-SiO2-500 (30% and 37%, respectively), although it is notably less pronounced. Therefore, the differences in the fitting results of the Ni-Al1-SiO2-500 component suggest that in Ni-Al5-SiO2-500 a large fraction of the Ni(II) sites are located in the vicinity of the strong BAS of the SiO2–AlOx interface, yet this is not the case in Ni-Al10-SiO2-500.
FTIR studies using pyridine (Py) as the probe molecule show that bands due to the pyridinium ion (Py protonated by strong BAS) are observed clearly in Ni-Al1-SiO2-500, while they are less intense in Ni-Al5-SiO2-500 and nearly not discernable in Ni-Al10-SiO2-500 (Fig. S13†). Those results are consistent with the degree of abundance of strong BAS in the respective Al1-, Al5- and Al10-SiO2-500 supports as discussed above.26 However, an additional Lewis acid site (LAS) emerges in Ni-Al1,5,10-SiO2-500 materials, identified by an adsorbed Py band at 1614 cm−1 that is lacking in Al1,5,10-SiO2-500 supports. This band is ascribed to Py coordinated to isolated Ni(II) sites (see ESI† for additional discussion).
The catalytic performance of the prepared Ni–Al catalysts was assessed by flowing 10% C2H4/N2 through a catalyst bed that was kept at 350 °C (space velocity was 200 mL gcat−1 h−1). While all three catalysts are active for ETP, their initial activity, stability with TOS and distribution of products differ significantly (Table 2). Ni-Al1-SiO2-500, a material with the most abundant strong BAS, converts ethene notably faster than Ni-Al5-SiO2-500 and Ni-Al10-SiO2-500 catalysts; the respective initial (at TOS = 7 min) consumption rates of ethene are 16.8, 5.9 and 6.4 g(C2H4) gNi−1 h−1 obtained at 55%, 20% and 30% conversion, respectively (Table 2, Fig. S14†). Interestingly, in contrast to Ni-Al1-SiO2-500 and Ni-Al10-SiO2-500, Ni-Al5-SiO2-500 displays an activation period (ca. 150 min) associated with an increase of ethene conversion from ca. 20% to 25%, before deactivation sets in. Possible reasons for the activation period of Ni-Al5-SiO2-500 are discussed below. Deactivation with TOS is observed for all three catalysts. After 20 h TOS, the conversion of Ni-Al1-, Ni-Al5- and Ni-Al10-SiO2-500 decreases to only 8%, 10% and 3% of the initial values, respectively. The initial productivity to propene on Ni-Al1-SiO2-500 is 2.7 g(C3H6) gNi−1 h−1, followed by Ni-Al10-SiO2-500 and Ni-Al5-SiO2-500 (0.6 and 0.2 g(C3H6) gNi−1 h−1, respectively). However, the productivity to propene raises after 150 min TOS for Ni-Al5-SiO2-500 (due to an activation period mentioned above) reaching ca. 0.8 g(C3H6) gNi−1 h−1.
| Entry | Catalyst | Ni content (wt%) | Ethene conversion (%) | Propene productivity (g gNi−1 h−1) | C4 olefins productivity (g gNi−1 h−1) | C1–C4 alkane productivity (g gNi−1 h−1) | H2 productivity (g gNi−1 h−1) | Carbon balance (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Ni-Al1-SiO2-500 | 0.73 | 55 (8) | 2.7 (0.6) | 5.5 (1.8) | 1.1 (0.1) | 0.16 (0.005) | 80 (100) |
| 2 | Ni-Al5-SiO2-500 | 1.03 | 20 (10) | 0.2 (0.5) | 0.3 (1.3) | 0.2 (0.1) | 0.03 (0.003) | 88 (100) |
| 3 | Ni-Al10-SiO2-500 | 1.09 | 30 (3) | 0.6 (0.1) | 1.7 (0.4) | 0.4 (0.1) | 0.02 (0.004) | 89 (100) |
Butenes (1-butene, iso-butene, trans- and cis-2-butene) are formed on all three catalysts, consistent with the dimerization of ethene to 1-butene on the isolated Ni(II) sites followed by isomerization reactions (Fig. S16–S18†). In addition to olefins, the Ni-Al-SiO2-500 catalysts produce C1–C4 alkanes, in particular ethane, whereby the partial selectivity to ethane among the C1–C4 alkanes exceeds 85% (Fig. S21†). The productivity to alkanes is especially high at short TOS, suggesting the formation of hydrogen (via coking) and its use for the hydrogenation of alkenes and, possibly, hydrogenolysis of alkanes. For instance, at TOS = 50 min, the productivity to C1–C4 alkanes is 0.79, 0.52 and 0.30 g(alkanes) gNi−1 h−1 for Ni-Al1-, Ni-Al5- and Ni-Al10-SiO2-500, respectively (Fig. S20†). At TOS = ca. 1 h the productivity to alkanes starts to decrease for all three catalysts, before ceasing after TOS = ca. 10 h (Table 2, Fig. S16–S18†).
The initial carbon balance on Ni-Al1-, Ni-Al5-, and Ni-Al10-SiO2-500 is only 80%, 88% and 89%, respectively. However, after 10 h on stream, all three catalysts reach ca. 100% carbon balance (Table 2, Fig. S16–S18†). The low initial carbon balance indicates the formation of non-volatile hydrocarbons and coke. Coking is also evidenced by the change of color of the Ni-Al-SiO2-500 catalysts from pale-grey to black after the catalytic tests and is consistent with the increase of the catalyst mass observed during in situ TGA experiments (Fig. S33†). In addition, Raman spectra of spent Ni–Al catalysts reveal two main peaks centered at ca. 1350 cm−1 and 1580 cm−1, ascribed to the disordered carbon and ordered graphitic lattice, respectively (Fig. S32†).35 The ratio between these peaks, i.e., the D1/G area ratio, is used to evaluate the degree of disorder in the graphitic coke deposits. While similar D1/G ratio of 1.5 was obtained for Ni-Al1-SiO2-500 and Ni-Al5-SiO2-500, Ni-Al10-SiO2-500 features a lower D1/G ratio of 0.3 (see ESI† for further details). The evolution of the productivity to alkanes with TOS correlates closely with that of coking (evaluated by the carbon balance) and the formation of H2 (Fig. S20†).
Interestingly, aromatic products (benzene, toluene, xylenes, ethylbenzene, BTXE) form on all three Ni-Al-SiO2-500 catalysts. Ni-Al1-SiO2-500 exhibits the highest productivities to toluene and C8 aromatics (ethylbenzene and o-, m-, p-xylene) whereas Ni-Al10-SiO2-500 produces predominantly benzene at TOS = 7 min (Fig. S19†).36 After TOS = 8 h, the partial selectivity (i.e., the selectivity among aromatic products) to C8 aromatics is more than 60% on all three catalysts. Control experiments show that the respective supports Al1-, Al5-, and Al10-SiO2-500 do not catalyze ETP, nor do they form any aromatic products (Fig. S30†). The initial ethene conversion on these supports does not exceed 5% and is likely due to the formation of coke.
To understand the nature of the surface species formed during the ETP experiment, Ni-Al5-SiO2-500 was heated with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 13C2H4/C2H4 at 350 °C for 12 h. The subsequently acquired 13C CP-MAS spectrum of the resulted black solid shows two major peaks in the range 110–150 ppm and 10–40 ppm, assigned to aromatic carbonaceous species and aliphatic groups, respectively (Fig. S38†). The presence of such species has been ascribed previously to the formation of alkylated aromatic hydrocarbons in zeolites,37–39 and in other alumina-based catalysts.40 Furthermore, comparing the 1H spin-echo spectra of Ni-Al5-SiO2-500 after exposure to 13C2H4/C2H4 (350 °C, 12 h) with that of fresh Ni-Al5-SiO2-500, confirms the emergence of peaks at ca. 2 ppm and 7 ppm, consistent with benzylic and aromatic protons, respectively (Fig. S39†); the peak at ca. 21 ppm in 13C NMR spectrum further suggests the presence of Ar–CH3 benzylic groups (Fig. S38†). Notably, no additional signals are observed in the range 4–6 ppm, ruling out the presence of adsorbed olefinic species (i.e. ethene, propene, butenes, etc.). Analysis of the gas phase of the batch experiment by GC-MS confirms the formation of a statistical mixture of 13C isotopologues of propene (Fig. S41, Table S6†). In addition to propene, ethane is detected and its formation is explained by the hydrogenation of ethene by H2 (formed due to coking) over the course of the experiment. However, in contrast to propene, ethane features mostly a mixture of unlabelled and di-labelled isotopologues with only a small amount (ca. 5%) of the mono-labelled isotopologue (Fig. S40, Table S5†). This result is inconsistent with the presence of olefin metathesis based ETP mechanism that has been proposed previously for Ni-based catalysts,12 and is in line with the cracking pathways.23
1 mixture of 13C2H4/C2H4 at 350 °C for 12 h. The subsequently acquired 13C CP-MAS spectrum of the resulted black solid shows two major peaks in the range 110–150 ppm and 10–40 ppm, assigned to aromatic carbonaceous species and aliphatic groups, respectively (Fig. S38†). The presence of such species has been ascribed previously to the formation of alkylated aromatic hydrocarbons in zeolites,37–39 and in other alumina-based catalysts.40 Furthermore, comparing the 1H spin-echo spectra of Ni-Al5-SiO2-500 after exposure to 13C2H4/C2H4 (350 °C, 12 h) with that of fresh Ni-Al5-SiO2-500, confirms the emergence of peaks at ca. 2 ppm and 7 ppm, consistent with benzylic and aromatic protons, respectively (Fig. S39†); the peak at ca. 21 ppm in 13C NMR spectrum further suggests the presence of Ar–CH3 benzylic groups (Fig. S38†). Notably, no additional signals are observed in the range 4–6 ppm, ruling out the presence of adsorbed olefinic species (i.e. ethene, propene, butenes, etc.). Analysis of the gas phase of the batch experiment by GC-MS confirms the formation of a statistical mixture of 13C isotopologues of propene (Fig. S41, Table S6†). In addition to propene, ethane is detected and its formation is explained by the hydrogenation of ethene by H2 (formed due to coking) over the course of the experiment. However, in contrast to propene, ethane features mostly a mixture of unlabelled and di-labelled isotopologues with only a small amount (ca. 5%) of the mono-labelled isotopologue (Fig. S40, Table S5†). This result is inconsistent with the presence of olefin metathesis based ETP mechanism that has been proposed previously for Ni-based catalysts,12 and is in line with the cracking pathways.23
Transmission electron microscopy (TEM) imaging and energy dispersive X-ray (EDX) mapping of spent Ni-Al1-SiO2-500 (i.e., after 20 h TOS) reveal the formation of Ni NPs (Fig. S22†). This is explained by the evolution of Ni(II) sites to reduced Ni species under reaction conditions, their de-grafting (dissociation) from the support and agglomeration to Ni NPs (either metallic Ni, carburized Ni, or a mixture of thereof). Note that no Ni NPs are observed in any of the fresh catalysts (Fig. S4–S6†). Interestingly, in contrast to Ni-Al1-SiO2-500, no Ni NPs are found in spent Ni-Al5-SiO2-500 and Ni-Al10-SiO2-500; EDX mappings show that the Ni sites remain homogenously distributed on Ni-Al5-SiO2-500 and Ni-Al10-SiO2-500 after the 20 h catalytic test (Fig. S23 and S24†). Our observations, i.e. the formation of coke and the retention of isolated Ni sites on deactivated Ni-Al5-SiO2-500 and Ni-Al10-SiO2-500 catalysts, suggest that regeneration of the initial activity by calcination should be viable for these two catalysts.
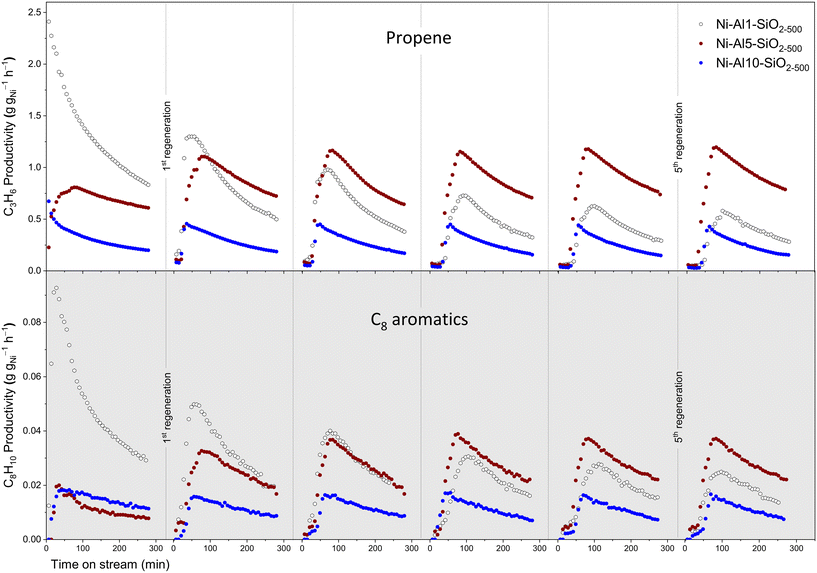
To test this hypothesis, the reaction–regeneration cycles were carried out by passing synthetic air at 500 °C for 1 h (to remove coke) through the catalyst bed that has been exposed for 5 h to ETP conditions. No substantial changes in the productivity of propene (except for the increased propene productivity after the first regeneration cycle for Ni-Al5-SiO2-500, vide infra) were observed between the first, second and indeed up to the fifth regeneration cycle for Ni-Al5-SiO2-500 and Ni-Al10-SiO2-500, suggesting that these catalysts can be fully regenerated (Fig. 2). However, regeneration of Ni-Al1-SiO2-500 could restore the propene productivity of the previous cycles only partially. Those observations further confirm the differences in the deactivation mechanisms between Ni-Al1-SiO2-500 on one hand and Ni-Al5- and Ni-Al10-SiO2-500 on the other hand, consistent with the TEM results. In other words, two deactivation pathways proceed in Ni-Al1-SiO2-500 during ETP, i.e. (i) the formation of Ni NPs due to the reduction of Ni(II) single sites and the agglomeration of the reduced species (see above, Fig. S22†) and (ii) coke deposition. While coke can be removed by calcination, the oxidation of the agglomerated Ni NPs gives NiO, which, in contrast to isolated sites of Ni(II), does not catalyze the oligomerization of ethene efficiently. In contrast, the Ni-Al5- and Ni-Al10-SiO2-500 catalysts deactivate only by coke deposition (i.e., blocking of the active sites by coke) since their catalytic activity is fully recovered after regeneration by calcination.
Interestingly, after the first regeneration cycle, Ni-Al5-SiO2-500 displays a propene productivity that is increased by ca. 30% and which is maintained in every subsequent ETP-regeneration cycle (Fig. 2). We note that the temperature used for regeneration is higher relative to the initial calcination temperature of the grafted Ni species (500 °C and 400 °C, respectively). This higher temperature is required because coke removal is incomplete at 400 °C, and as a result, a longer activation period and a lower maximum propene productivity is observed if a regeneration temperature of 400 °C is used instead of a regeneration temperature of 500 °C (Fig. S34†). However, if Ni-Al5-SiO2-500 is prepared by calcination of the grafted Ni species at 500 °C, the resulted propene productivities of the fresh and the regenerated catalysts are similar (Fig. S31†). Therefore, the observed increase by ca. 30% in the propene productivity of Ni-Al5-SiO2-500 may be due to a structural evolution of Ni-Al5-SiO2-500 at 500 °C.
To probe for possible structural changes of Ni-Al5-SiO2-500 during the regeneration process, we have applied 27Al MAS NMR spectroscopy. The narrower spectral lines in Ni-Al5-SiO2-500-regen reveal a higher structural ordering in this material, yet the lines become also narrower in the Ni-free reference Al5-SiO2-500-regen (Fig. S37†). In addition, the relative fractions of 4Al/5Al/6Al sites change in both Ni-Al5-SiO2-500-regen and Al5-SiO2-500-regen (Table S4†). The modelling of Ni-Al5-SiO2-500-regen shows smaller Δδiso and ![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) Q than in fresh Ni-Al5-SiO2-500 (both modellings use three Al sites). In addition, a shift of
Q than in fresh Ni-Al5-SiO2-500 (both modellings use three Al sites). In addition, a shift of ![[small delta, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c5.gif) iso towards higher chemical shifts and a decreased proportion of four-coordinated aluminum environments is observed after regeneration. This points to a complex mechanism for the structural reorganization during regeneration involving not only the OH groups (usually at the origin of the coordination increase), but also the chemical Al/Si mixing, which drives changes in
iso towards higher chemical shifts and a decreased proportion of four-coordinated aluminum environments is observed after regeneration. This points to a complex mechanism for the structural reorganization during regeneration involving not only the OH groups (usually at the origin of the coordination increase), but also the chemical Al/Si mixing, which drives changes in ![[small delta, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c5.gif) iso.26 Differences in the NMR parameters of Al5-SiO2-500-regen and Ni-Al5-SiO2-500-regen show that Ni also plays a role in the structural ordering of the catalysts during regeneration.
iso.26 Differences in the NMR parameters of Al5-SiO2-500-regen and Ni-Al5-SiO2-500-regen show that Ni also plays a role in the structural ordering of the catalysts during regeneration.
The activation time that lasts ca. 50–100 min is observed in all Ni-Al-SiO2-500 catalysts following regeneration; in addition, fresh Ni-Al5-SiO2-500 features an activation period as well. After 90 min TOS, following the sixth regeneration, i.e. close to the highest propene productivity, the three Ni–Al catalysts were collected and FTIR spectra were recorded without exposure to air. The spectra of fresh and regenerated catalysts after 90 min TOS are similar (Fig. S25†). That being said, the comparison between the Py-FTIR spectra of fresh and regenerated Ni-Al5-SiO2-500 catalysts reveals a decreased relative intensity of the IR band corresponding to the Lewis acidic Ni(II) sites as compared to the intensity of Py on the Al-based LAS on the regenerated catalysts (Fig. S35†). This result suggests that restructuring of the catalyst surface after calcination may occur with TOS, although more studies are needed to understand details of this process. Lastly, TEM images and EDX mapping of the activated and spent Ni-Al-SiO2-500 catalysts are also similar, i.e. Ni NPs are only observed in activated and spent Ni-Al1-SiO2-500 and not observed in Ni-Al5-SiO2-500 and Ni-Al10-SiO2-500 (Fig. S27–S29†).
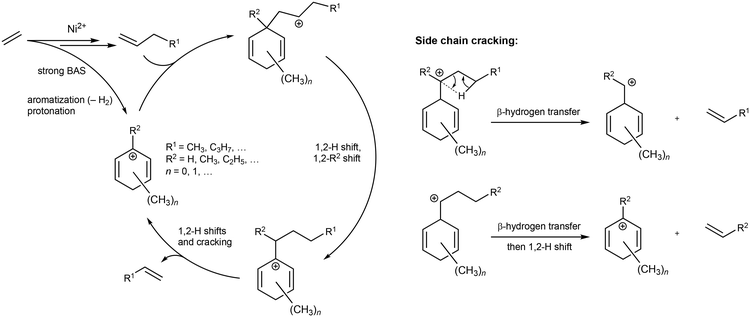
Interestingly, comparing changes in the productivity to aromatic products with TOS, in particular C8 aromatics, with changes in the productivity to propene reveal a very clear correlation (Fig. 2 and S15†). For instance, the continuously decreasing propene productivity on Ni-Al1-SiO2-500 with every additional regeneration cycle correlates closely with the decreasing productivity to C8 aromatics. This result suggests that the formation of propene may involve the intermediacy of alkylated aromatics (and, possibly, vice versa). The results discussed above are consistent with the following reaction mechanism. Ethene is first dimerized to 1-butene and then oligomerized further on Ni(II) sites,41 whereas strong BAS catalyze olefin aromatization to BTXE,42 and provide protonated aromatic species that can be alkylated by olefins such as 1-butene. Cracking of the side chain of the resulting intermediates may form propene and regenerate the cationic aromatic species (Scheme 1). This carbon-pool mechanism is thus complementary to the ETP mechanisms proposed previously (chain growth on Ni sites and cracking of oligomers on the strong BAS),22 and is related to the pathways of the zeolite-catalyzed ETP reaction.28,29
In conclusion, we have reported that Ni-based catalysts with abundant strong BAS show a higher propene productivity relative to catalysts with less abundant strong BAS. The role of the strong BAS is not only limited to the cracking of ethene oligomers, but they also catalyze the aromatization of ethene and higher olefins to BTXE products. When protonated, these aromatics can be further alkylated, which in turn facilitates the cracking process (i.e., the carbon-pool mechanism). Lastly, the formation of Ni aluminate sites on the amorphous alumina overlayer prevents Ni(II) from overreduction which would lead to the formation of Ni nanoparticles. These findings expand the scope and understanding of Ni-based catalysts in the ETP reaction. Our future work will focus on the development of isolated Ni(II) sites that are stable under ETP conditions in the presence of abundant strong BAS. Moreover, kinetic studies will be needed to improve our understanding of the role of the cracking of linear oligomers vs. the cracking of alkylated aromatic species in the ETP reaction.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Scientific Centre for Optical and Electron Microscopy (ScopeM, ETH Zürich) for providing access to electron microscopy facilities. Z. C. thanks ETH Zürich for financial support (ETH-40 17-2). S. R. D. and C. C. acknowledge the Swiss National Science Foundation (grants 200021_169134, 200020B_192050, and CRSII5_183495). The Swiss Norwegian Beamlines (SNBL) at European Synchrotron Facilities (ESRF) is acknowledged for providing access to the BM31 beamline. This publication was created as part of NCCR Catalysis (grant number 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation.References
- J. S. Plotkin, Catal. Today, 2005, 106, 10–14 CrossRef CAS
.
-
H. A. Wittcoff, B. G. Reuben and J. S. Plotkin, Industrial Organic Chemicals, John Wiley & Sons, Inc., Hoboken, New Jersey, 2nd edn, 2004 Search PubMed
.
- S. R. Docherty, L. Rochlitz, P. A. Payard and C. Copéret, Chem. Soc. Rev., 2021, 50, 5806–5822 RSC
.
- S. Chen, X. Chang, G. Sun, T. Zhang, Y. Xu, Y. Wang, C. Pei and J. Gong, Chem. Soc. Rev., 2021, 50, 3315–3354 RSC
.
- A. Galadima and O. Muraza, Ind. Eng. Chem. Res., 2015, 54, 4891–4905 CrossRef CAS
.
- U. Olsbye, M. Bjørgen, S. Svelle, K.-P. Lillerud and S. Kolboe, Catal. Today, 2005, 106, 108–111 CrossRef CAS
.
- M. A. Ali, S. Ahmed, N. Al-Baghli, Z. Malaibari, A. Abutaleb and A. Yousef, Catal. Lett., 2019, 149, 3395–3424 CrossRef CAS
.
- C. Copéret, F. Allouche, K. W. Chan, M. P. Conley, M. F. Delley, A. Fedorov, I. B. Moroz, V. Mougel, M. Pucino, K. Searles, K. Yamamoto and P. A. Zhizhko, Angew. Chem., Int. Ed., 2018, 57, 6398–6440 CrossRef PubMed
.
- B. Lin, Q. Zhang and Y. Wang, Ind. Eng. Chem. Res., 2009, 48, 10788–10795 CrossRef CAS
.
- M. Taoufik, E. Le Roux, J. Thivolle-Cazat and J. M. Basset, Angew. Chem., Int. Ed., 2007, 46, 7202–7205 CrossRef CAS PubMed
.
- E. Mazoyer, K. C. Szeto, N. Merle, J. Thivolle-Cazat, O. Boyron, J.-M. Basset, C. P. Nicholas and M. Taoufik, J. Mol. Catal., 2014, 385, 125–132 CrossRef CAS
.
- M. Iwamoto and Y. Kosugi, J. Phys. Chem. C, 2007, 111, 13–15 CrossRef CAS
.
- K. Ikeda, Y. Kawamura, T. Yamamoto and M. Iwamoto, Catal. Commun., 2008, 9, 106–110 CrossRef CAS
.
- T. Lehmann, T. Wolff, V. M. Zahn, P. Veit, C. Hamel and A. Seidel-Morgenstern, Catal. Commun., 2011, 12, 368–374 CrossRef CAS
.
- A. S. Frey and O. Hinrichsen, Microporous Mesoporous Mater., 2012, 164, 164–171 CrossRef CAS
.
- M. Tanaka, A. Itadani, Y. Kuroda and M. Iwamoto, J. Phys. Chem. C, 2012, 116, 5664–5672 CrossRef CAS
.
- T. Lehmann, T. Wolff, C. Hamel, P. Veit, B. Garke and A. Seidel-Morgenstern, Microporous Mesoporous Mater., 2012, 151, 113–125 CrossRef CAS
.
- L. Alvarado Perea, T. Wolff, P. Veit, L. Hilfert, F. T. Edelmann, C. Hamel and A. Seidel-Morgenstern, J. Catal., 2013, 305, 154–168 CrossRef CAS
.
- M. Stoyanova, M. Schneider, M.-M. Pohl and U. Rodemerck, Catal. Commun., 2017, 92, 65–69 CrossRef CAS
.
- M. Iwamoto, K. Kasai and T. Haishi, ChemSusChem, 2011, 4, 1055–1058 CrossRef CAS PubMed
.
- M. Iwamoto, Molecules, 2011, 16, 7844–7863 CrossRef CAS
.
- I. B. Moroz, A. Lund, M. Kaushik, L. Severy, D. Gajan, A. Fedorov, A. Lesage and C. Copéret, ACS Catal., 2019, 9, 7476–7485 CrossRef CAS
.
- U. Rodemerck, E. V. Kondratenko, M. Stoyanova and D. Linke, J. Catal., 2020, 389, 317–327 CrossRef CAS
.
- A. R. Mouat, C. George, T. Kobayashi, M. Pruski, R. P. van Duyne, T. J. Marks and P. C. Stair, Angew. Chem., Int. Ed., 2015, 54, 13346–13351 CrossRef CAS PubMed
.
- A. G. M. Rankin, P. B. Webb, D. M. Dawson, J. Viger-Gravel, B. J. Walder, L. Emsley and S. E. Ashbrook, J. Phys. Chem. C, 2017, 121, 22977–22984 CrossRef CAS PubMed
.
- M. Kaushik, C. Leroy, Z. Chen, D. Gajan, E. Willinger, C. R. Müller, F. Fayon, D. Massiot, A. Fedorov, C. Copéret, A. Lesage and P. Florian, Chem. Mater., 2021, 33, 3335–3348 CrossRef CAS
.
- C. Coperet, A. Comas-Vives, M. P. Conley, D. P. Estes, A. Fedorov, V. Mougel, H. Nagae, F. Nunez-Zarur and P. A. Zhizhko, Chem. Rev., 2016, 116, 323–421 CrossRef CAS PubMed
.
- K. Lee and S. B. Hong, ACS Catal., 2019, 9, 10640–10648 CrossRef CAS
.
- W. Dai, X. Sun, B. Tang, G. Wu, L. Li, N. Guan and M. Hunger, J. Catal., 2014, 314, 10–20 CrossRef CAS
.
- P. Tian, Y. Wei, M. Ye and Z. Liu, ACS Catal., 2015, 5, 1922–1938 CrossRef CAS
.
- S. Ilias and A. Bhan, ACS Catal., 2012, 3, 18–31 CrossRef
.
- K. Horioka, K. Takahashi, N. Morimoto, H. Horiuchi, M. Akaogi and S. Akimoto, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1981, 37, 635–638 CrossRef
.
-
B. K. Teo, EXAFS: Basic Principles and Data Analysis, Springer-Verlag, Berlin, Heidelberg, New York, Tokyo, 1986 Search PubMed
.
- J. Timoshenko and A. Kuzmin, Comput. Phys. Commun., 2009, 180, 920–925 CrossRef CAS
.
- A. Sadezky, H. Muckenhuber, H. Grothe, R. Niessner and U. Pöschl, Carbon, 2005, 43, 1731–1742 CrossRef CAS
.
- C. Wang, X. Zhao, M. Hu, G. Qi, Q. Wang, S. Li, J. Xu and F. Deng, Angew. Chem., Int. Ed., 2021, 60, 23630–23634 CrossRef CAS PubMed
.
- P. Ferri, C. Li, C. Paris, A. Vidal-Moya, M. Moliner, M. Boronat and A. Corma, ACS Catal., 2019, 9, 11542–11551 CrossRef CAS
.
- J. T. C. Wennmacher, S. Mahmoudi, P. Rzepka, S. Sik Lee, T. Gruene, V. Paunovic and J. A. van Bokhoven, Angew. Chem., Int. Ed., 2022, 61, e202205413 CrossRef CAS PubMed
.
- J. P. Lange, A. Gutsze, J. Allgeier and H. G. Karge, Appl. Catal., 1988, 45, 345–356 CrossRef CAS
.
- M. A. Callejas, M. T. Martínez, T. Blasco and E. Sastre, Appl. Catal., A, 2001, 218, 181–188 CrossRef CAS
.
- E. Koninckx, P. S. F. Mendes, J. W. Thybaut and L. J. Broadbelt, Appl. Catal., A, 2021, 624, 118296 CrossRef CAS
.
- E. A. Uslamin, H. Saito, N. Kosinov, E. Pidko, Y. Sekine and E. J. M. Hensen, Catal. Sci. Technol., 2020, 10, 2774–2785 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cy01272c |
| This journal is © The Royal Society of Chemistry 2022 |