Synthesis of adiponitrile from dimethyl adipate and ammonia in the vapor-phase over niobium oxide†
Abstract
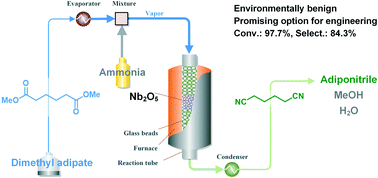
An environmentally benign route leading to adiponitrile (ADN), a nylon-6,6 intermediate, was achieved by direct vapor-phase nitrilation of dimethyl adipate (DMA) with ammonia (NH3) in a fixed-bed reactor. The challenges of this reaction include not only inhibiting both intramolecular cyclization and the intermolecular polycondensation of the electron-withdrawing group functionalized carbon chain but also suppressing the thermolysis of aliphatic DMA and ADN. Both amphoteric oxides and acidic oxides enable the transformation but exhibit different reactivities. Under evaluation conditions, the acidic oxides exhibit moderate to good nitrile selectivity, while the amphoteric oxides exhibit high DMA conversions with relatively low nitrile selectivity. Under the optimized conditions, the desired ADN was obtained with a selectivity of 84.3% with 97.7% DMA conversion over Nb2O5-1000 consisting of a monoclinic structure/phase (H-Nb2O5). The structure–performance relationship of this catalyst was preliminarily discussed. Furthermore, a tentative reaction pathway consisting of ammonolysis and dehydration processes in a tandem mode was proposed.


 Please wait while we load your content...
Please wait while we load your content...