Engineering the activity of amine dehydrogenase in the asymmetric reductive amination of hydroxyl ketones†
Abstract
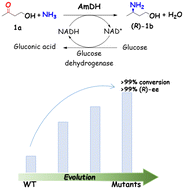
Chiral amino alcohols are essential structural motifs required in pharmaceutical agents and bioactive natural products. Our previous work explored a series of engineered amine dehydrogenases (AmDHs) derived from amino acid dehydrogenases (AADHs), enabling the asymmetric reductive amination of hydroxyl ketones to chiral amino alcohols. The catalytic efficiency towards γ-hydroxyl ketones, however, is modest in comparison to the activity with vicinal ones. Taking the AmDH from Geobacillus stearothermophilus (GsAmDH) as a template, a rational design campaign was performed with 4-hydroxybutan-2-one serving as the model substrate. Several variants with improved catalytic efficiency were obtained after three rounds of mutagenesis. Compared with the starting enzyme, the conversion of the whole cell reaction containing the engineered mutants was increased from 45% up to 99% in the transformation of 4-hydroxybutan-2-one into (R)-3-amino-1-butanol. The best variant mh174 bearing seven substitutions, showed a 4.2-fold increase in catalytic efficiency (kcat/Km) compared to that of the starting enzyme, while retaining a high enantioselectivity (ee > 99%). In an upscaled reaction (100 mM substrate), mh174 led to 99% conversion of the (R)-product within 36 h and 90% yield. The engineered variants also enable the asymmetric reductive amination of a broad range of hydroxyl ketones, including aliphatic, aromatic, and alicyclic ketones. Overall, this study expands the AmDH toolbox and provides useful variants that may be further developed in the synthesis of high-value chiral amino alcohols.


 Please wait while we load your content...
Please wait while we load your content...