Well-defined manganese complex catalyzed dehydrogenative synthesis of quinazolin-4(3H)-ones and 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides†
Abstract
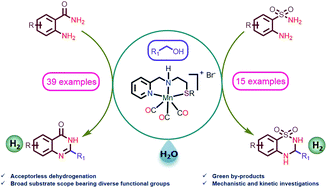
The synthesis of N-heterocycles has been considered an emerging topic of chemical research due to their widespread usage in medicinal chemistry, materials science, and natural product synthesis. The development of green, atom economical and sustainable strategies to construct N-heterocycles employing readily available renewable starting materials is a high priority for the scientific community. Alcohol is an economical and widely abundant greener substrate derived from a diverse range of sustainable resources. Earth-abundant 3d-transition metal catalyzed acceptorless dehydrogenative coupling (ADC) displays an environmentally benign synthetic approach to construct N-heterocycles by liberating eco-friendly by-products, water and hydrogen. Herein, we described the first Mn-catalyzed synthesis of quinazoline-4(3H)-ones and 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides via acceptorless dehydrogenative annulation. This method features the usage of various primary alcohols including benzyl alcohol bearing tolerable functional groups, heteroaryl and aliphatic alcohols as coupling partners. The synthetic value of this procedure is shown by the efficient construction of 2-(quinolin-2-yl)quinazolin-4(3H)-one, which is an intermediate for luotonins A, B, and E. Detailed mechanistic investigations and kinetic monitoring were performed to understand the reaction sequence and the role of the catalyst in different steps.


 Please wait while we load your content...
Please wait while we load your content...