Open Access Article
Open Access ArticleKinetic studies on Lewis acidic metal polyesterification catalysts – hydrolytic degradation is a key factor for catalytic performance†
Lukas A.
Wolzak
 ab,
Rogier
van Gemert
c,
Keimpe J.
van den Berg
c,
Joost N. H.
Reek
ab,
Rogier
van Gemert
c,
Keimpe J.
van den Berg
c,
Joost N. H.
Reek
 *b,
Moniek
Tromp
*b,
Moniek
Tromp
 *ad and
Ties J.
Korstanje
*ad and
Ties J.
Korstanje
 *a
*a
aSustainable Materials Characterization, van 't Hoff Institute for Molecular Sciences (HIMS), University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands. E-mail: t.j.korstanje@uva.nl
bBio-inspired, Homogeneous and Supramolecular Catalysis, van ‘t Hoff Institute for Molecular Sciences (HIMS), University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands. E-mail: j.n.h.reek@uva.nl
cAkzo Nobel Car Refinishes BV, Rijksstraatweg 31, 2171 AJ Sassenheim, The Netherlands
dFaculty of Science and Engineering, Materials Chemistry — Zernike Institute for Advanced Materials, University of Groningen Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: moniek.tromp@rug.nl
First published on 11th March 2022
Abstract
Kinetic analysis of polyesterification reactions using Lewis-acidic metal catalysts have been performed. While Sn-based catalysts are superior to Ti-based catalysts under neat polycondensation conditions (high [H2O]), the result is inverted under azeotropic conditions (low [H2O]). These findings show that the catalytic activity is crucially determined by the robustness of the catalyst against hydrolytic degradation.
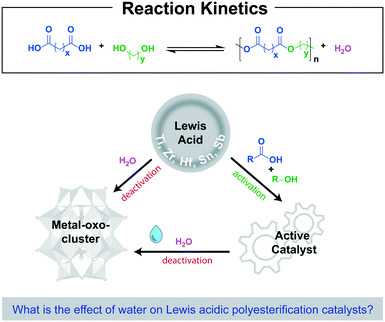
In the past decades, extensive advances in the production of monomers for polyesters, derived from renewable resources, have been made.1–7 Nevertheless, the synthesis of polyesters itself still heavily relies on well-known condensation reactions, such as the direct esterification of alcohols and carboxylic acids. This route remains a highly sustainable method to obtain polyesters, given the high atom efficiency and water being the only by-product.8 However, due to the harsh reaction conditions required for this reaction, the use of a catalyst that can reduce the reaction temperature is preferred. High-valent Lewis acidic metal catalysts based on group 4 metals (Ti, Zr and Hf), Sn and Sb have displayed distinct activity in direct polyesterification reactions.9–13 Yet, potent Lewis acidic metal catalysts based on abundant and non-toxic metals, that are active in direct polyesterification are still highly desired. Next to the ability to lower the activation energy of a reaction, an effective catalyst should be fairly robust, since deactivation of the catalyst can dominate the observed activity. Lewis acidic metal catalysts are typically deactivated by water, which is formed during the initial esterification step. The reaction with water affords the formation of metal-oxo-clusters with reduced catalytic activity (Scheme 1). Hydrolysis of both group 4 metal alkoxides, and mono- and di-alkyl tin(IV) complexes is well-known and results in the formation of a wide variety of metal-oxo-clusters.14–18 It remains however unclear to date to what extent these metal-oxo-clusters also form during polyesterification reactions. Polyesterification reactions catalyzed by group 4 metals are predominantly reported under azeotropic conditions in combination with removal of water by a scavenger.19,20 Such dehydrating agents drive the equilibrium towards the ester product, and prevent prolonged exposure of the catalyst to water formed during polyesterification. Furthermore, multidentate ligands are employed to reduce the number of hydrolysable terminal ligands, which hampers uncontrolled cluster formation.21–25
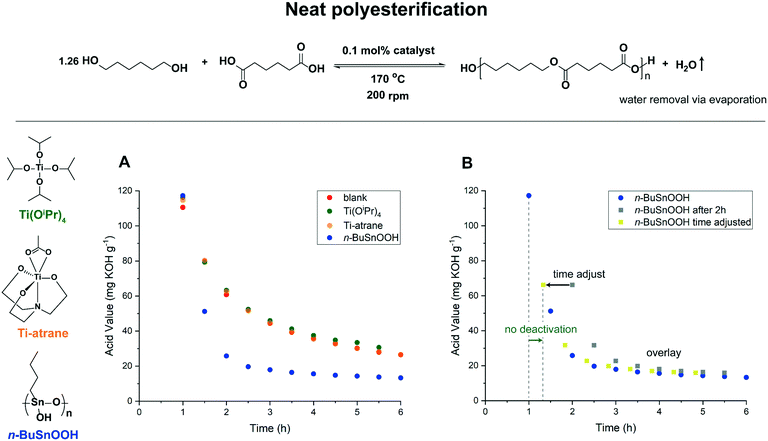
Although it is clear that hydrolytic degradation of Lewis acidic catalysts can be problematic, the degree of deactivation will depend on the applied reaction conditions such as polarity of the reaction mixture, reaction temperature, use of solvent and reduced pressure.26–29 This prompted us to investigate the robustness of different commercially applied polyesterification catalysts through analysis of the reaction profile.29 Herein, we report our kinetic investigations on titanium- and n-butylstannoic acid-catalyzed polyesterification reactions under neat polycondensation and azeotropic conditions. We started our investigation with the neat polyesterification of 1,6-hexanediol, in excess, and adipic acid in the presence of 0.1 mol% catalyst (relative to –OH groups) at 170 °C to synthesize polyester-diols. All formed water was collected in a Dean-Stark apparatus (Fig. S1†).
In order to obtain kinetic profiles, the reaction progress was followed via the acid value (AV) determined by titration. Three different catalysts were selected: n-BuSnOOH, Ti(OiPr)4 and titanium(triethanolaminato)acetate (Ti-atrane) (Fig. 1). In general, catalysts were added after 1 hour reaction time at 170 °C to ensure effective removal of formed reaction water before catalyst addition. Under neat conditions, n-BuSnOOH (Fig. 1A) displays catalytic activity, with an AV = 13.3 mg KOH g−1 after 6 hours. This in contrast to Ti(OiPr)4 and Ti-atrane which are not catalytically active, and perform comparable to the reaction without metal catalyst (blank reaction). To obtain insight in the robustness of n-BuSnOOH under neat polycondensation conditions we turned to reaction progress kinetic analysis (RPKA).30 More specifically, same-excess experiments can help to disclose deactivation of the catalysts via the overlay of kinetic reaction profiles. Because the polyesterification reaction is also catalyzed by the carboxylic acid, which results in a background reaction, catalyst deactivation via same-excess conditions can be determined via the addition of catalyst at a later reaction point in time (Fig. 1B). Next to the initial n-BuSnOOH-catalyzed polyesterification, a similar reaction was performed, but now with addition of n-BuSnOOH after 2 hours reaction time (Fig. 1B, orange trace). Upon catalyst addition, a clear increase in rate is observed and within 5 hours the AV resembles the value observed in the initial n-BuSnOOH catalyzed reaction (Fig. S2A†). The time-adjusted profile of the second experiment (Fig. 1B, yellow trace) gives a good overlay with the initial experiment and is indicative for the absence of catalyst deactivation. Next, we turned to the polyesterification of oligomers analogue to a two-step polycondensation method. Although high water concentrations present in the initial stage of the polyesterification reaction were circumvented, the titanium-based catalysts still performed poorly (Fig. S3A†). In addition, the use of more apolar oligomers resulted only in minor activity for the titanium-based catalysts (Fig. S3B†).
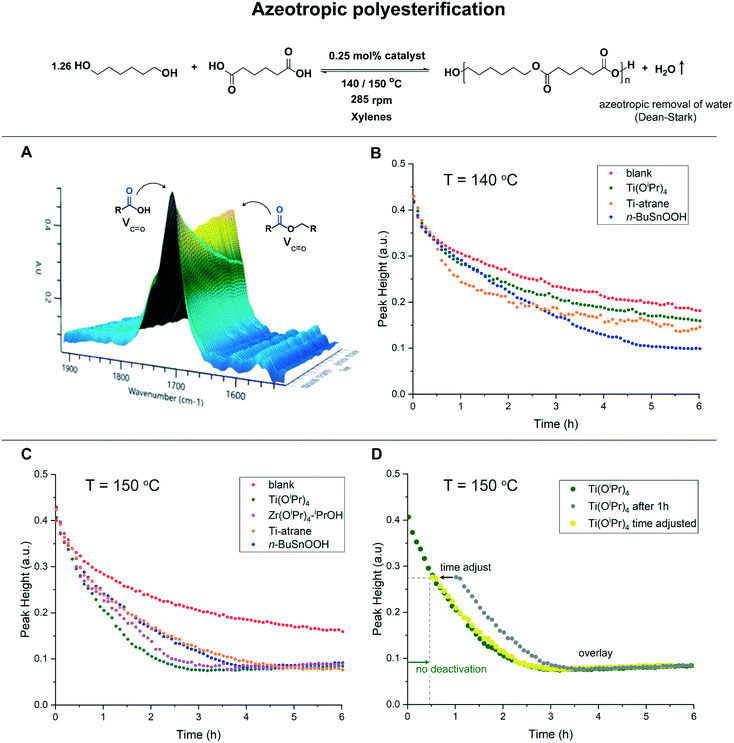
We hypothesize that the lack of catalytic activity for the titanium-based catalysts results from deactivation by water, hence a harmful water concentration is present in the reaction mixture (Table S1†). We therefore turned to azeotropic reaction conditions, which enable the effective continuous removal of stoichiometrically formed water. Under these conditions, the polyesterification of 1,6-hexanediol and adipic acid was performed with the inclusion of xylene as high-boiling solvent, in the presence of 0.25 mol% (relative to –OH groups) of catalyst (Fig. 2). The course of the reaction was followed with in situ infrared spectroscopy via the decrease of the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of the carboxylic acid over time (Fig. 2A), which correlates well with determined acid values (Fig. S10†). We observed that under azeotropic reaction conditions at 140 °C, n-BuSnOOH still has the highest activity after 6 hours. However, the Ti-atrane and Ti(OiPr)4 catalysts perform significantly better than the blank reaction (Fig. 2B). Remarkably, the Ti-atrane catalyst outperforms n-BuSnOOH in the first two hours of the reaction. In addition, after 1 hour the reaction mixture became turbid, which is indicative for the formation of titanium-oxo-clusters and thus the deactivation of Ti(OiPr)4 (Fig. S3†).
O vibration of the carboxylic acid over time (Fig. 2A), which correlates well with determined acid values (Fig. S10†). We observed that under azeotropic reaction conditions at 140 °C, n-BuSnOOH still has the highest activity after 6 hours. However, the Ti-atrane and Ti(OiPr)4 catalysts perform significantly better than the blank reaction (Fig. 2B). Remarkably, the Ti-atrane catalyst outperforms n-BuSnOOH in the first two hours of the reaction. In addition, after 1 hour the reaction mixture became turbid, which is indicative for the formation of titanium-oxo-clusters and thus the deactivation of Ti(OiPr)4 (Fig. S3†).
Analysis of the H2O concentration revealed water levels between 2286–4669 ppm after 6 hours reaction time (Table S2†). However, an increase in the reaction temperature to 150 °C proved to be effective to ensure an azeotropic reflux over the whole reaction period, i.e. the water concentrations were significantly diminished to 158–523 ppm for all metal-catalyzed reactions (Table S2†).30 Performing the reaction at 150 °C resulted in Ti(OiPr)4 and Zr(OiPr)4–iPrOH being very active catalyst (Fig. 2C).31 The improved activity of Group 4 alkoxides encouraged us to investigate the robustness of Ti(OiPr)4 under azeotropic reflux conditions at 150 °C. An additional same excess experiment was performed, with catalyst addition at a later point in time, which indicated no catalyst deactivation (Fig. 2D). Overall, the robustness of the catalyst tends to be crucial, which is highlighted by the superior performance of n-BuSnOOH. To further test the robustness, against hydrolytic degradation, of the different catalysts we investigated the hydrolysis of a polyester (AV = 24.6 mg KOH g−1) of 1,6-hexanediol and adipic acid.32–38 After 2 hours reaction time, in an autoclave at 150 °C, (Fig. S12†) an AV of 81.9 mg KOH g−1 was found for the n-BuSnOOH catalyzed reaction. Whereas the blank reaction and Ti-atrane catalyzed reaction resulted in significantly lower AV's with 35.3 and 33.4 mg KOH g−1 (Table S3†). These results unveil that n-BuSnOOH has indeed the ability to the withstand high water concentrations that accumulate under neat polyesterification and polyester hydrolysis conditions. Moreover, these findings are in agreement with our recent spectroscopic study on n-BuSnOOH catalyzed esterification, in which we demonstrated that at a relevant reaction temperature (90 °C), the polymeric n-BuSnOOH forms a monomeric n-butyltintricarboxylate and remains unaffected by water.39 In contrast to n-BuSnOOH, the titanium catalysts greatly suffer from hydrolytic degradation under neat polyesterification conditions. The Ti-atrane complex shows only minor activity for example. However, when the water concentrations are significantly lowered via azeotropic removal, the group 4 metal alkoxides turned out to being very active catalysts, outperforming n-BuSnOOH in terms of activity. Nevertheless, deactivation via hydrolytic degradation is facile for all titanium-based catalysts as demonstrated by the azeotropic experiments at 140 °C. The utilization of a multidentate ligand, i.e. in the Ti-atrane complex, resulted in a slightly improved water tolerance. On the other hand, the Ti-atrane performs worse than Ti(OiPr)4 under optimal azeotropic conditions (150 °C), probably because the ligand effectively shields the active metal center which hampers catalysis.
Conclusions
We have demonstrated, that n-BuSnOOH is an active and robust catalyst under neat polyesterification conditions. This is in contrast to Ti(OiPr)4 and Ti-atrane, which only gave minor initial activity. Low water concentrations (<550 ppm), which can be achieved via azeotropic removal, proved to be pivotal for group 4 metal alkoxides to be active and robust catalysts. These findings reveal the detrimental effect of water on titanium-based catalysts, even for complexes that bear multidentate ligands. This demonstrates that the robustness of the Lewis acidic metal catalyst dictates the performance in many polyesterification reactions. We believe that these insights will propel the sustainable production of (biobased) polyesters via catalysis by non-toxic and abundant Lewis acidic metals.Author contributions
This work is part of the Advanced Research Center for Chemical Building Blocks, ARC CBBC, which is co-founded and co-financed by the Netherlands Organization for Scientific Research (NWO, contract 736.000.000) and the Netherlands Ministry of Economic Affairs and Climate.Conflicts of interest
The authors declare no conflict of interest.Notes and references
- H. Sardon, D. Mecerreyes, A. Basterretxea, L. Avérous and C. Jehanno, ACS Sustainable Chem. Eng., 2021, 9, 10664–10677 CrossRef CAS.
- O. Gómez-Jiménez-Aberasturi and J. R. Ochoa-Gómez, J. Chem. Technol. Biotechnol., 2017, 92, 705–711 CrossRef.
- K. Lang, R. J. Sánchez-Leija, R. A. Gross and R. J. Linhardt, Polymers, 2020, 12, 1–25 CrossRef.
- I. Hevus, N. G. Ricapito, S. Tymoshenko, S. N. Raja and D. C. Webster, ACS Sustainable Chem. Eng., 2020, 8, 5750–5762 CrossRef CAS.
- A. F. Sousa, C. Vilela, A. C. Fonseca, M. Matos, C. S. R. Freire, G. J. M. Gruter, J. F. J. Coelho and A. J. D. Silvestre, Polym. Chem., 2015, 6, 5961–5983 RSC.
- Q. Zhang, M. Song, Y. Xu, W. Wang, Z. Wang and L. Zhang, Prog. Polym. Sci., 2021, 120, 101430 CrossRef CAS.
- J. Iglesias, I. Martínez-Salazar, P. Maireles-Torres, D. Martin Alonso, R. Mariscal and M. López Granados, Chem. Soc. Rev., 2020, 49, 5704–5771 RSC.
- J. Otera and J. Nishikido, Esterification. Methods, Reactions, and Applications, Wiley-VCH Verlag GmbH & Co., Weinheim, 2nd edn, 2010 Search PubMed.
- K. Ishihara, Tetrahedron, 2009, 65, 1085–1109 CrossRef CAS.
- U. K. Thiele, Int. J. Polym. Mater., 2001, 50, 387–394 CrossRef CAS.
- W. A. MacDonald, Polym. Int., 2002, 51, 923–930 CrossRef CAS.
- L. Papadopoulos, A. Zamboulis, N. Kasmi, M. Wahbi, C. Nannou, D. A. Lambropoulou, M. Kostoglou, G. Z. Papageorgiou and D. N. Bikiaris, Green Chem., 2021, 23, 2507–2524 RSC.
- M. R. Meneghetti and S. M. P. Meneghetti, Catal. Sci. Technol., 2015, 5, 765–771 RSC.
- V. G. Kessler, The Synthesis and Solution Stability of Alkoxide Precursors. In: Handbook of Sol-Gel Science and Technology, ed. L. Klein, M. Aparicio and A. Jitianu, Springer, Cham., 2018 Search PubMed.
- U. Schubert, J. Mater. Chem., 2005, 15, 3701–3715 RSC.
- U. Schubert, Acc. Chem. Res., 2007, 40, 730–737 CrossRef CAS.
- T. J. Boyle, R. P. Tyner, T. M. Alam, B. L. Scott, J. W. Ziller and B. G. Potter, J. Am. Chem. Soc., 1999, 121, 12104–12112 CrossRef CAS.
- V. Chandrasekhar, S. Nagendran and V. Baskar, Coord. Chem. Rev., 2002, 235, 1–52 CrossRef CAS.
- K. Ishihara, S. Ohara and H. Yamamoto, Science, 2000, 290, 1140–1142 CrossRef CAS.
- K. Ishihara, M. Nakayama, S. Ohara and H. Yamamoto, Tetrahedron, 2002, 58, 8179–8188 CrossRef CAS.
- R. Eckert, D. Runkel, V. Voerckel and J.-P. Wiegner, US Pat., US2012/0316316A1, 2012 Search PubMed.
- W. M. P. B. Menge and J. G. Verkade, Inorg. Chem., 1991, 30, 4628–4631 CrossRef CAS.
- A. A. D. Tulloch, A. Cooper and R. H. Duncan, US Pat., US2010/0292449A1, 2010 Search PubMed.
- J. A. Bander, S. D. Lazarus and I. C. Twilley, US Pat., US1981/4260735, 1981 Search PubMed.
- L. A. Wolzak, J. I. van der Vlugt, K. J. van den Berg, J. N. H. Reek, M. Tromp and T. J. Korstanje, ChemCatChem, 2020, 12, 5229–5235 CrossRef CAS.
- E. Leverd, F. Fradet and A. Maréchal, Eur. Polym. J., 1987, 23, 695–698 CrossRef.
- E. Leverd, F. Fradet and A. Maréchal, Eur. Polym. J., 1987, 23, 699–704 CrossRef.
- F. Leverd, A. Fradet and E. Maréchal, Eur. Polym. J., 1987, 23, 705–709 CrossRef CAS.
- N. Jacquel, F. Freyermouth, F. Fenouillot, A. A. Rousseau, J. Pascault, P. Fuertes and R. Saint-Loup, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5301–5312 CrossRef CAS.
- D. G. Blackmond, J. Am. Chem. Soc., 2015, 137, 10852–10866 CrossRef CAS PubMed.
- See Figure S11 for discussion on the peak height minimum of the Ti(OiPr)4-catalyzed reaction in Figure 2C and D.
- A. Sakakura, S. Nakagawa and K. Ishihara, Tetrahedron, 2006, 62, 422–433 CrossRef CAS.
- A. Sakakura, Y. Koshikari and K. Ishihara, Tetrahedron Lett., 2008, 49, 5017–5020 CrossRef CAS.
- A. Sakakura, H. Watanabe, S. Nakagawa and K. Ishihara, Chem. – Asian J., 2007, 2, 477–483 CrossRef CAS.
- K. Ishihara, S. Nakagawa and A. Sakakura, J. Am. Chem. Soc., 2005, 127, 4168–4169 CrossRef CAS.
- A. Sakakura, S. Nakagawa and K. Ishihara, Nat. Protoc., 2007, 2, 1746–1751 CrossRef CAS PubMed.
- Y. Koshikari, A. Sakakura and K. Ishihara, Org. Lett., 2012, 14, 3194–3197 CrossRef CAS PubMed.
- A. Sakakura, Y. Koshikari, M. Akakura and K. Ishihara, Org. Lett., 2012, 14, 30–33 CrossRef CAS PubMed.
- L. A. Wolzak, J. J. Hermans, F. de Vries, K. J. van den Berg, J. N. H. Reek, M. Tromp and T. J. Korstanje, Catal. Sci. Technol., 2021, 11, 3326–3332 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cy02306c |
| This journal is © The Royal Society of Chemistry 2022 |