Electronically tuneable orthometalated RuII–NHC complexes as efficient catalysts for C–C and C–N bond formations via borrowing hydrogen strategy†
Praseetha Mathoor
Illam
and
Arnab
Rit
 *
*
Department of Chemistry, Indian Institute of Technology Madras, Chennai-600036, India. E-mail: arnabrit@iitm.ac.in
First published on 10th November 2021
Abstract
The catalytic activities of a series of simple and electronically tuneable cyclometalated RuII–NHC complexes (2a–d) were explored in various C–C/N bond formations following the borrowing hydrogen process. Slight modifications in the ligand backbone were noted to tune the activities of these complexes. Among them, the complex 2d featuring a 1,2,4-triazolylidene donor with a 4-NO2–phenyl substituent displayed the highest activity for the coupling of diverse secondary and primary alcohols with a low catalyst loading of 0.01 mol% and a sub-stoichiometric amount of inexpensive KOH base. The efficacy of this simple system was further showcased in the challenging one-pot unsymmetrical double alkylation of secondary alcohols using different primary alcohols. Moreover, the complex 2d also effectively catalyses the selective mono-N-methylation of various aromatic and aliphatic primary amines using methanol to deliver a range of N-methyl amines. Mechanistically, the β-alkylation reaction follows a borrowing hydrogen pathway which was established by the deuterium labelling experiment in combination with various control experiments. Intriguingly, in situ1H NMR and ESI-MS analyses evidently suggested the involvement of a Ru–H species in the catalytic cycle and further, the kinetic studies revealed a first order dependence of the reaction rate on the catalyst as well as the alcohol concentrations.
1. Introduction
The development of easily accessible and inexpensive ligands for the preparation of effective homogeneous catalysts is a trending research area in modern chemistry. In this direction, N-heterocyclic carbenes (NHCs) have been established as versatile ligands as they are capable of coordinating different metal ions in various oxidation states to yield various efficient catalysts.1–5 Further, the ease of preparation coupled with the easy stereoelectronic tunability via simple topological alterations6–8 has made them appealing in various research fields.4,5,9 Among various late transition metal–NHC complexes, ruthenium complexes have ensured a distinctive position in homogeneous catalysis because of their ability in catalysing diversified organic transformations (such as metathesis, (transfer)hydrogenation, oxidation, synthesis of enol-esters and heterocyclic compounds, hydrofunctionalization, etc.) and their cost-effectiveness as compared to other precious transition metal complexes.10–13 Among them, chelated RuII–NHC complexes have been recognized as one of the most effective catalysts in majority of these organic transformations.14–19 Thus, various possibilities to modulate the stereoelectronics of chelating NHC ligands and, in turn, the performances of their transition metal complexes have started to emerge recently. These modifications mainly include the alterations in the carbene donors (imidazolylidene, ImNHC; triazolylidene, tzNHC; mesoionic carbene, MIC; etc.) and/or the substituents on the azolium moieties.20–25 Variations of either the NHC donors and/or the substituents at the ligand backbone have been detected to have a substantial influence on the stereoelectronics and thus, the catalytic activities of their metal complexes.15,20,23,26,27Along with the development of efficient catalytic systems, the current socio-economic scenario requires sustainable reaction methodologies in synthetic chemistry. In this line, the hydrogen auto-transfer (borrowing hydrogen, BH) strategy has received immense attention for the construction of various C–C/N bonds utilising less reactive and readily available alcohols as potential alkylating agents due to its high atom economy and greener nature (releasing water as the sole by-product).28–31 Pioneered by the independent studies of Grigg32 and Watanabe33 in the C–N bond formation (1981) and by Cho and co-workers in the β-alkylation of secondary alcohols (2003),34 transition metal catalysed borrowing hydrogen protocols have witnessed significant progress offering improved catalytic efficiency in recent years.29,31 Along this line, NHC-based late transition metal systems including ruthenium–NHC complexes have been explored widely for the C–N bond forming reactions such as the N-alkylation of amines and oxidative amide synthesis,16,24,35–38 however, related catalytic C–C bond formation reactions are less explored. The early example in the area of RuII–NHC catalysed C-alkylation protocols was by Peris et al. (2007) who utilized a di-ruthenium complex connected by a triazolediylidene (ditz) ligand for the β-alkylation of secondary alcohols (Fig. 1).39 Later, in 2009, a pyrimidine substituted imidazolylidene supported cyclometalated mono-ruthenium complex was detected to deliver a similar activity for the same reaction.40 Despite further progress in the area of β-alkylation of secondary alcohols, most of the reported systems require higher catalyst loading and stoichiometric and/or expensive bases.39–42 In a recent contribution in this area, we have observed that the introduction of the 1,2,4-triazolylidene donor in a dicyclometalated RuII-system exhibits excellent activity at low catalyst and base loadings in the β-alkylation reaction but requires two RuII-centres.43
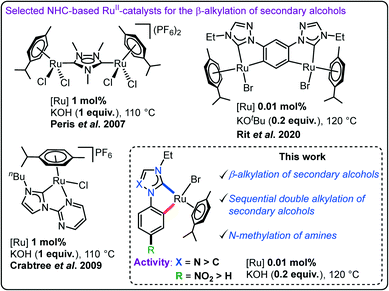 | ||
| Fig. 1 Selected examples of RuII–NHC catalysts reported for the β-alkylation of secondary alcohols with primary alcohols following a borrowing hydrogen strategy and the present work. | ||
Motivated by these observations along with our experience in tuning the catalytic activity of RuII–NHC complexes via stereoelectronic alterations, we hypothesised that suitable modifications in ancillary NHC-ligands might allow us to attain high activity using a simpler catalyst system. With our continued effort in this direction, we herein present easily accessible simple imidazolylidene (ImNHC) and 1,2,4-triazolylidene (tzNHC) based cyclometalated RuII–NHC complexes (2a–d) as efficient catalysts for various C–C/N bond forming reactions. A change in the carbene donor (complex 2avs.2b) as well as the introduction of a para-NO2-group at the N-phenyl wingtip of the complex 2c/d significantly improved their catalytic activities in the coupling of secondary and primary alcohols with a low catalyst loading of 0.01 mol% and inexpensive KOH base. The applicability of the present system is further showcased in the challenging unsymmetrical double alkylation of secondary alcohols in one pot with two different primary alcohols to access the corresponding α,α-disubstituted ketones. Further, the catalyst 2d was also found to be effective for the N-methylation of various amines using methanol as the green methylating agent.
2. Results and discussion
2.1. Synthesis and catalytic applications of the RuII–NHC complexes 2a–d
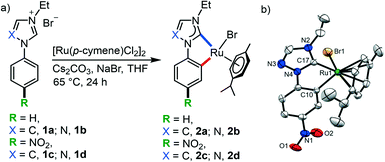
The cyclometalated complex 2d along with the reported complexes 2a–c15,44 were synthesized from the respective azolium salts 1a–d following a deprotonation and metalation pathway (Scheme 1). Formation of the complex 2d was confirmed by NMR (Fig. S1 and S2†) and ESI-MS as well as X-ray crystallographic studies. The electronic nature of the complexes was assessed with the help of the ruthenium bound CNHC signals in the 13C{1H} NMR spectra. These resonances (2a: 187.0, 2b: 189.3, 2c: 189.2, 2d: 192.1 ppm) manifest that the complex 2d has the most electron deficient RuII-center among these complexes which is in agreement with the electronic nature detected for previous RuII–NHC complexes.15,44,45 With these well-defined and electronically tuneable CNHC^C cyclometalated complexes, we planned to study their activities in green tandem organic transformations. Accordingly, we first chose the coupling of the readily available lignocellulosic secondary and primary alcohols following the borrowing hydrogen strategy due to its greener nature when compared to the traditional C–C bond forming reactions using mutagenic alkyl halides.46At the outset, the activity of the complexes 2a–d was studied in the β-benzylation of 1-phenylethanol using benzyl alcohol. The optimization of the reaction conditions was performed with 0.01 mol% of 2a–d and KOH base (0.2 equiv.) in toluene (Table 1). Among the unsubstituted NHC complexes 2a–b, the triazolylidene complex 2b provided the quantitative conversion of 1-phenylethanol to 5a and 5a′ (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) in 12 h, whereas the imidazolylidene complex 2a offered only 90% conversion with a similar selectivity towards the β-alkylated alcohol 5a (entry 2 vs. 1). Further, the reduction of the reaction duration to 8 h resulted in a significant decrease in the conversion of 1-phenylethanol (70–81%) to the alkylated products (entries 3–4) although complex 2b still performed better than 2a.
4) in 12 h, whereas the imidazolylidene complex 2a offered only 90% conversion with a similar selectivity towards the β-alkylated alcohol 5a (entry 2 vs. 1). Further, the reduction of the reaction duration to 8 h resulted in a significant decrease in the conversion of 1-phenylethanol (70–81%) to the alkylated products (entries 3–4) although complex 2b still performed better than 2a.
| Entry | Catalyst | Time (h) | Conversion (%) | Selectivity 5a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a′ (%) 5a′ (%) |
|---|---|---|---|---|
| a Reaction conditions: 1-phenylethanol (1 mmol), benzyl alcohol (1.1 mmol), KOH (0.2 mmol), catalyst (0.0001 mmol), toluene (1 mL), conversion based on GC-MS. b 0.0005 mmol of PPh3 was added.47 c Isolated yield. d Reaction carried out without base. | ||||
| 1 | 2a | 12 | 90 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 2 | 2b | 12 | 100 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 3 | 2a | 8 | 70 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 4 | 2b | 8 | 81 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 5 | 2a | 6 | 87 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 6 | 2b | 6 | 98 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 7 | 2c | 8 | 90 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 8 | 2d | 8 | 99 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4 (92%) 4 (92%)
|
| 9d | 2d | 8 | Traces | — |
The above results demonstrate that the complex 2b featuring a poor σ-donor, 1,2,4-triazolylidene, is a better precatalyst than its analogue 2a with an imidazolylidene donor. To substantiate this trend, the activities of the complexes 2c–d with an electron withdrawing NO2-substituent at the para-position of the N-phenyl ring were investigated for the selected reaction with the assumption that an electron deficient RuII-complex might perform better. Indeed, the complex 2c outperformed the complexes 2a–b and provided 90% conversion of 3a (entry 7 vs. 3/4) with an excellent selectivity (96%) towards 5a in 8 h. Importantly, the combination of a 1,2,4-triazolylidene donor with an NO2-substituent in 2d yielded the best precatalyst (resulting in an essentially quantitative conversion of 3a with 92% isolated yield of 5a) among the complexes 2a–d. It should be noted that the present system is among the most active Ru-based systems reported so far. Further, various bases and solvents were also screened and KOH and toluene were observed to be the most suitable base and solvent, respectively, for this catalytic system (Tables S1 and S2, ESI†). Additionally, both catalyst and base are necessary for this reaction as evidenced from the poor catalytic outcome in their absence. Finally, the reaction duration was set to 12 h for further studies of the substrate scope to maintain the high product yield/selectivity.
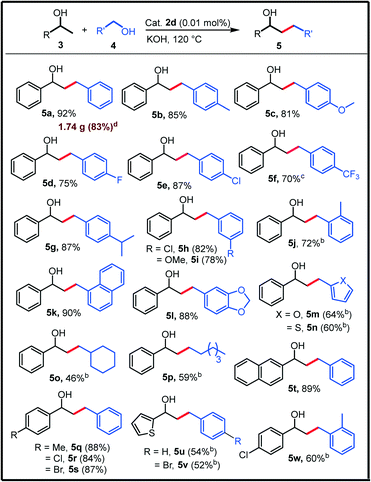
With the identified optimum reaction conditions, we explored the scope of the present protocol by varying both the coupling partners (secondary and primary alcohols, Table 2). Various primary alcohols with electron donating as well as the electron withdrawing substituents at the para-position successfully delivered the corresponding β-alkylated alcohols 5b–g in good to excellent yields (upto 87%) and no dehalogenation was observed for the halogenated primary alcohols 4d–e. Strong electron withdrawing p-CF3 substituted benzyl alcohol is also active which delivered the respective product 5f in decent yield. Further, the primary alcohols with meta-substituents also coupled effectively (5h–i) whereas the sterically congested ortho-methyl substituted benzyl alcohol was found to be relatively less active (5j). However, 1-naphthyl methanol and 3,4-(methylenedioxy)benzyl alcohol could be utilised as effective alkylating agents to provide the corresponding products (5k–l) in excellent yields. Further, the heteroaromatic primary alcohols such as 1-furanmethanol and 1-thiophenemethanol were also observed to be active (5m and 5n). Next, 1-phenylethanol was successfully alkylated with challenging aliphatic primary alcohols such as cyclohexylmethanol and 1-hexanol to obtain the products (5o–p) in decent yields. After that, various secondary alcohols were reacted with benzyl alcohol, which delivered the corresponding β-alkylated alcohols (5q–t) in excellent yields (84–89%). The coupling of heteroaromatic secondary alcohol 1-(thiophen-2-yl)ethan-1-ol with (substituted)benzyl alcohols also provided the corresponding products 5u–v. Finally, the sterically crowded 2-methylbenzyl alcohol was employed for the β-alkylation of 1-(4-chlorophenyl)ethanol, which delivered the product 5w in 60% yield. Satisfactorily, a high turnover number (TON) of 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved with a lower catalyst loading of 10 ppm (0.001 mol%). Moreover, the practicality of this protocol was showcased by applying it for a large-scale reaction (10 mmol scale) and the compound 5a was isolated in 83% yield (1.74 g).
000 was achieved with a lower catalyst loading of 10 ppm (0.001 mol%). Moreover, the practicality of this protocol was showcased by applying it for a large-scale reaction (10 mmol scale) and the compound 5a was isolated in 83% yield (1.74 g).
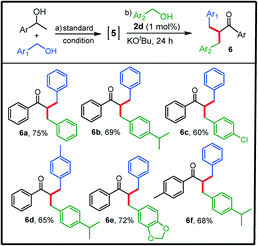
We further explored the aptness of the present catalyst system in challenging sequential double alkylation of secondary alcohols in one pot as the produced dialkylated compounds find widespread applications in organic synthesis and the pharmaceutical industry.46 Recently, the monoalkylation of methylene ketones using alcohols for accessing α,α-disubstituted ketones was achieved by employing various transition metal catalysts.48–52 However, only a few studies on the three-component one-pot double alkylation of methyl ketones, inspired by the initial work of Donohoe et al.,49via sequential addition of primary alcohols have been reported.48,53,54 Although the area of ketone α-alkylation has now been developed considerably, studies on the synthesis of α,α-disubstituted ketones via double alkylation of secondary alcohols using different primary alcohols in one pot are scarce.55,56 Given the very high activity of our simple cyclometalated RuII–NHC complexes in the β-alkylation process, we motivated ourselves to investigate the activity of 2d in the above-mentioned transformation. Gladly, the complex 2d was found to be effective (1 mol% loading) for this transformation also and the scope of this reaction was explored by reacting selected 1-phenylethanol derivatives with various primary alcohols containing electron-donating and electron-withdrawing substituents and the corresponding unsymmetrically disubstituted ketones 6a–f were isolated in overall good yields (up to 75%, Table 3).
2.2. Selective mono-methylation of amines
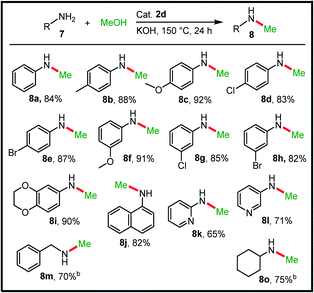
To study the generality of this simple catalyst system, we further studied the efficacy of the complex 2d in the selective mono methylation of various amines using methanol as a C1-source to obtain diverse N-methylated amines. They are essential components of various pharmaceuticals, agrochemicals, detergents, dyes, etc., and frequently encountered as synthetic intermediates.57,58 Even though this strategy is highly desirable because of its greener nature when compared to traditional methylation processes which use toxic methyl halides, diazomethanes, etc., it still remains challenging due to the higher activation energy (e.g. ΔH = +84 and +68 kJ mol−1 for the dehydrogenation of methanol and ethanol, respectively) and the possibility of dimethylation.59,60 To date, although various late transition metal-based protocols have been developed for this transformation, there have been relatively few ruthenium-based protocols.24,60–63 Inspired by the success of the present system in alkylation processes using alcohols, we then considered the N-methylation reaction and to our delight, the catalyst 2d with 1 mol% loading successfully mono-methylated various (hetero)aromatic and aliphatic amines in the presence of KOH base (Table 4).Firstly, the methylation of aniline selectively provided the N-methylaniline, 8a, in good yield of 84% and no dimethylation was observed. Other aniline derivatives with electron-donating and electron-withdrawing substituents at both the para- and meta-positions also selectively delivered the corresponding mono-methylated compounds in good to excellent yields (8b–h, up to 92% yield). Significantly, no dehalogenation was observed as evident from the high yields (82–87%) of the halogen containing N-methylated amine products (8d–e and 8g–h). Further, the 1,4-benzodioxole substituted aniline and α-naphthylamine are also active which furnished the secondary amines, 8i–j, in excellent yields (82–90%). More challenging heteroaromatic primary amines were also cleanly converted to the mono-methylated products (8k–l) in moderate yields and the reactivity of 3-aminopyridine was noted to be slightly better than that of 2-aminopyridine possibly due to chelating nature of 2-aminopyridine. Additionally, a couple of aliphatic primary amines were also examined and satisfactorily, mono-methylation of these substrates occurred effectively. Thus, the present system selectively provides diverse mono-N-methylated amines in high yields from a wide range of primary amines and methanol and the activity is also comparable with other reported RuII-based systems.24,60,62
2.3. Investigation of the reaction mechanism
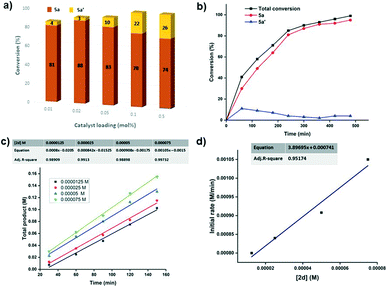
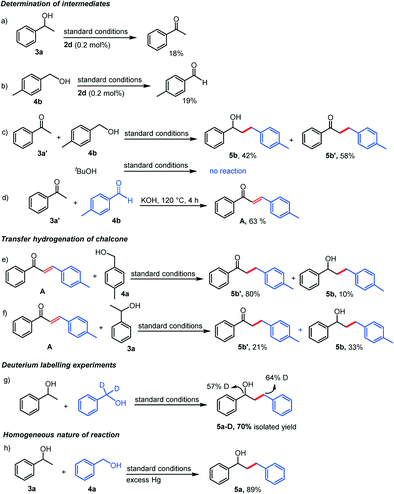
Several control experiments were then undertaken to probe the plausible reaction mechanism for the β-alkylation process. Along this line, we first studied the reaction between 1-phenylethanol (3a) and benzyl alcohol (4a) with various catalyst loadings (Fig. 2a) to learn the effect of catalyst loading on the reaction selectivity (ketone vs. alcohol), if any. The reaction proceeds to completion within 4 h when higher catalyst loadings (0.1–0.5 mol%) are used but the selectivity towards the desired β-alkylated alcohol product 5a was noted to be poor (74–78%). Meanwhile, the use of a lower catalyst loading of 0.05 mol% resulted in a higher selectivity towards 5a (89%) with a 93% total conversion of 3a. With further lowering of the catalyst loadings (0.02–0.01 mol%), the selectivity towards 5a increased up to 96% even though the reaction did not advance to completion in 4 h. All these observations suggest that the lower catalyst loading probably preclude the possible dehydrogenation of 5a to 5a′ during the course of the reaction. Further, a reaction temperature of 120 °C and above is necessary for the completion of the reaction (Fig. S84†). Next, we focused on the involvement of various reactive intermediates and initially studied the dehydrogenation of the starting alcohols. 1-Phenylethanol/4-methylbenzyl alcohol under our standard reaction conditions using 0.2 mol% of 2d yielded ∼18% of the corresponding carbonyl compounds via acceptorless dehydrogenation (Scheme 2a and b). Further, the reaction of acetophenone, the dehydrogenation product of 1-phenylethanol, with 4-methylbenzyl alcohol resulted in a complete consumption of acetophenone within 4 h to yield 5b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5b′ in 42
5b′ in 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 ratio (Scheme 2c). On the other hand, no reaction was observed when tBuOH was used as the coupling partner which underpins the requirement of an α-hydrogen in the reactants (Scheme 2c). Further, the reaction of acetophenone with 4-methylbenzaldehyde in the presence of KOH at 120 °C provided 1-phenyl-3-(p-tolyl)prop-2-en-1-one (A) in 63% isolated yield supporting the base promoted cross aldol reaction (Scheme 2d). Next, the reduction of the chalcone intermediate A with 4-methylbenzylalcohol/1-phenylethanol as the hydrogen source yielded a mixture of 5b and 5b′ (Scheme 2e and f). All the above observations indicate the formation of an α,β-unsaturated ketone intermediate from the reactant alcohols via dehydrogenation followed by aldol condensation during the catalytic process which is then reduced by the generated hydrogen/hydride species (formed during the dehydrogenation of starting alcohols, Scheme 2a and b) to yield the final product. Thus, the overall process follows a borrowing hydrogen (BH) pathway.
58 ratio (Scheme 2c). On the other hand, no reaction was observed when tBuOH was used as the coupling partner which underpins the requirement of an α-hydrogen in the reactants (Scheme 2c). Further, the reaction of acetophenone with 4-methylbenzaldehyde in the presence of KOH at 120 °C provided 1-phenyl-3-(p-tolyl)prop-2-en-1-one (A) in 63% isolated yield supporting the base promoted cross aldol reaction (Scheme 2d). Next, the reduction of the chalcone intermediate A with 4-methylbenzylalcohol/1-phenylethanol as the hydrogen source yielded a mixture of 5b and 5b′ (Scheme 2e and f). All the above observations indicate the formation of an α,β-unsaturated ketone intermediate from the reactant alcohols via dehydrogenation followed by aldol condensation during the catalytic process which is then reduced by the generated hydrogen/hydride species (formed during the dehydrogenation of starting alcohols, Scheme 2a and b) to yield the final product. Thus, the overall process follows a borrowing hydrogen (BH) pathway.
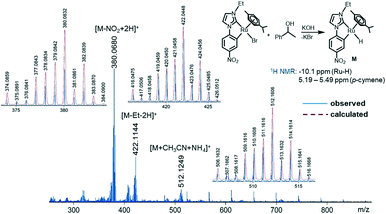
To have further evidence for a BH mechanism, deuterium labelling experiment was performed by reacting 1-phenylethanol with benzyl alcohol–D2 which provided the product 5a–D in 70% isolated yield (Scheme 2g). The 1H NMR analysis of 5a–D showed that deuterium is present at the α- and γ- position (57–64%) supporting the reaction pathway of alcohol dehydrogenation followed by the reduction of the generated chalcone intermediate, signifying a BH process. To examine whether any Ru–H species, normally entailed in the mechanism of a borrowing hydrogen process, is involved in our present protocol or not, complex 2c, 1-phenylethanol, and KOH were reacted for 2 h. The 1H-NMR spectrum of the reaction mixture displaying a resonance at δ −10.10 ppm which is very characteristic of a Ru–H species was observed (Fig. S89†) and the ESI-MS spectrum further supports its formation by exhibiting various relevant monopositive fragments (Fig. 3). The composition of the generated Ru–H species is further confirmed by the reaction in the presence of excess arenes (p-cymene and 1,3,5-trimethoxybenzene, 40 equiv. w.r.t. 2d) which indicates that the irreversible loss of the p-cymene moiety in the active catalyst is quite unlikely as there was no change in the catalytic outcome. Additionally, monitoring of the reaction progress as a function of time (Fig. 2b) discloses the absence of any chalcone intermediate and a very low concentration of 5a′ throughout the course of the reaction which asserts faster reduction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond than the C
C bond than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the α,β-unsaturated ketone intermediate. Further, kinetic studies employing the initial rate method for the 2d catalysed β-alkylation of 1-phenylethanol with benzyl alcohol were performed (Fig. 2c and d). The initial rate of the total product formation was noted to increase linearly (slope = 3.89695 M min−1) with a first order dependence on the concentration of 2d (0.0000125–0.000075 M). Additionally, the reaction also follows first order kinetics with respect to secondary alcohols (Fig. S86†). Finally, the homogeneous nature of the reaction was supported by mercury poisoning experiment (3 equiv. of Hg w.r.t. 3a) which yielded 5a in 89% (Scheme 2h). Moreover, the green metrics calculations revealed 88.7% reaction mass efficiency and 92.2% atom economy for the β-alkylation process (see ESI†) implying the benefits of this methodology from an environmental perspective.
O bond in the α,β-unsaturated ketone intermediate. Further, kinetic studies employing the initial rate method for the 2d catalysed β-alkylation of 1-phenylethanol with benzyl alcohol were performed (Fig. 2c and d). The initial rate of the total product formation was noted to increase linearly (slope = 3.89695 M min−1) with a first order dependence on the concentration of 2d (0.0000125–0.000075 M). Additionally, the reaction also follows first order kinetics with respect to secondary alcohols (Fig. S86†). Finally, the homogeneous nature of the reaction was supported by mercury poisoning experiment (3 equiv. of Hg w.r.t. 3a) which yielded 5a in 89% (Scheme 2h). Moreover, the green metrics calculations revealed 88.7% reaction mass efficiency and 92.2% atom economy for the β-alkylation process (see ESI†) implying the benefits of this methodology from an environmental perspective.
Based on our mechanistic investigation, a plausible mechanism for the alcohol cross coupling reaction is proposed (Scheme 3). The reaction commences with the dehalogenation of complex 2d with the formation of intermediates I and IIvia coordination of the corresponding alkoxides of 3a and 4a, respectively. The formation of the alkoxy species was validated by the ESI-MS spectrometric analysis of the reaction mixture (Fig. S91†). The intermediates I/II then undergo β-hydride elimination to yield a ruthenium hydride species III and the corresponding carbonyl compounds 3a′/4a′, supported by the 1H NMR spectrum of the reaction mixture and dehydrogenation experiments. In the next step, the hydride function of III is transferred to the α,β-unsaturated compound 5a*, formed via cross aldol condensation between 3a′ and 4a′, to produce the product 5a through 5a′ in the presence of 3a/4a. We believe that the second alkylation for the formation of 6a–f also follows a similar mechanism.
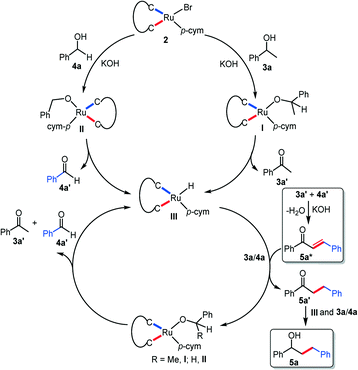 | ||
| Scheme 3 Plausible catalytic cycle for the β-alkylation of secondary alcohols following a borrowing hydrogen pathway. | ||
3. Conclusion
In conclusion, we have uncovered a series of simple and electronically tuneable cyclometalated RuII–NHC complexes 2a–d, obtained by varying the carbene donors and/or backbone substituents, in combination with inexpensive KOH base as efficient catalyst systems for the β-alkylation of diverse secondary alcohols via the borrowing hydrogen protocol. Intriguingly, the most electron deficient ruthenium complex 2d possessing a 1,2,4-triazolylidene donor with a 4-NO2–phenyl substituent was found to exhibit the highest activity among the complexes under consideration (2a–d) with a low catalyst loading of 0.01 mol%. The precatalyst 2d also effectively catalyses the challenging unsymmetrical double alkylation of secondary alcohols in a one-pot manner and the selective monomethylation of diverse amines. Various control experiments along with the kinetic and deuterium labelling studies certainly confirmed a borrowing hydrogen pathway involving a Ru–H intermediate, evidenced from the 1H NMR and ESI-mass analyses. We believe that this study involving the easily accessible and electronically tuneable simple catalyst systems 2a–d with manifold catalytic applications would certainly help in broadening the area of RuII–NHC catalysed borrowing hydrogen processes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We sincerely acknowledge SERB, India (Grant No. CRG/2020/000780) for the financial support. P. M. I thanks IIT Madras for a research fellowship. We gratefully acknowledge the instrument facility at the Department of Chemistry, IIT Madras.Notes and references
- F. E. Hahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122–3172 CrossRef CAS PubMed.
- S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef PubMed.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS.
- H. D. Velazquezd and F. Verpoort, Chem. Soc. Rev., 2012, 41, 7032–7060 RSC.
- M. Jalal, B. Hammouti, R. Touzani, A. Aounity and I. Ozdemir, Mater. Today: Proc., 2020, 31, S122–S129 CAS.
- H. Jacobsen, A. Correa, A. Poater, C. Costabile and L. Cavallo, Coord. Chem. Rev., 2009, 253, 687–703 CrossRef CAS.
- S. Díez-González and S. P. Nolan, Coord. Chem. Rev., 2007, 251, 874–883 CrossRef.
- T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 6940–6952 CrossRef.
- W. Wang, L. Cui, P. Sun, L. Shi, C. Yue and F. Li, Chem. Rev., 2018, 118, 9843–9929 CrossRef CAS.
- V. Dragutan, I. Dragutan, L. Delaude and A. Demonceau, Coord. Chem. Rev., 2007, 251, 765–794 CrossRef CAS.
- X. Ma, S. G. Guillet, M. Peng, K. Van Hecke and S. P. Nolan, Dalton Trans., 2021, 50, 3959–3965 RSC.
- Y. Mutoh, K. Yamamoto, Y. Mohara and S. Saito, Chem. Rec., 2021, 21, 1–14 CrossRef.
- L. Kathuria and A. G. Samuelson, Eur. J. Inorg. Chem., 2020, 2372–2379 CrossRef CAS.
- C. Gandolfi, M. Heckenroth, A. Neels, G. Laurenczy and M. Albrecht, Organometallics, 2009, 28, 5112–5121 CrossRef CAS.
- S. Bauri, S. N. R. Donthireddy, P. M. Illam and A. Rit, Inorg. Chem., 2018, 57, 14582–14593 CrossRef CAS PubMed.
- C. S. Tiwari, P. M. Illam, S. N. R. Donthireddy and A. Rit, Chem. – Eur. J. DOI:10.1002/chem.202102540.
- F. P. Malan, E. Singleton, P. H. van Rooyen, M. Albrecht and M. Landman, Organometallics, 2019, 38, 2624–2635 CrossRef CAS.
- A. Kozłowska, M. Dranka, J. Zachara, E. Pump, C. Slugovc, K. Skowerski and K. Grela, Chem. – Eur. J., 2014, 20, 14120–14125 CrossRef.
- S. Yadav, I. Dutta, S. Saha, S. Das, S. K. Pati, J. Choudhury and J. K. Bera, Organometallics, 2020, 39, 3212–3223 CrossRef CAS.
- P. M. Illam, S. N. R. Donthireddy, S. Chakrabartty and A. Rit, Organometallics, 2019, 38, 2610–2623 CrossRef CAS.
- J. C. Bernhammer, G. Frison and H. V. Huynh, Chem. – Eur. J., 2013, 19, 12892–12905 CrossRef CAS PubMed.
- S. N. Sluijter and C. J. Elsevier, Organometallics, 2014, 33, 6389–6397 CrossRef CAS.
- S. K. Gupta, S. K. Sahoo and J. Choudhury, Organometallics, 2016, 35, 2462–2466 CrossRef CAS.
- S. N. R. Donthireddy, P. M. Illam and A. Rit, Inorg. Chem., 2020, 59, 1835–1847 CrossRef CAS PubMed.
- A. Bolje, S. Hohloch, M. van der Meer, J. Košmrlj and B. Sarkar, Chem. – Eur. J., 2015, 21, 6756–6764 CrossRef CAS.
- S. Semwal, I. Mukkatt, R. Thenarukandiyil and J. Choudhury, Chem. – Eur. J., 2017, 23, 13051–13057 CrossRef CAS PubMed.
- S. Bauri, A. Mallik and A. Rit, Organometallics, 2020, 39, 3362–3374 CrossRef.
- A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410–1459 CrossRef CAS PubMed.
- T. Irrgang and R. Kempe, Chem. Rev., 2019, 119, 2524–2549 CrossRef CAS PubMed.
- S. N. R. Donthireddy, V. K. Pandey and A. Rit, J. Org. Chem., 2021, 86, 6994–7001 CrossRef CAS PubMed.
- S. N. R. Donthireddy, C. S. Tiwari, S. Kumar and A. Rit, Asian J. Org. Chem., 2021, 10, 464–484 CrossRef CAS.
- R. Grigg, T. R. B. Mitchell, S. Sutthivaiyakit and N. Tongpenyai, J. Chem. Soc., Chem. Commun., 1981, 611–612 RSC.
- Y. Watanabe, Y. Tsuji and Y. Ohsugi, Tetrahedron Lett., 1981, 22, 2667–2670 CrossRef CAS.
- C. S. Cho, B. T. Kim, H.-S. Kim, T. J. Kim and S. C. Shim, Organometallics, 2003, 22, 3608–3610 CrossRef CAS.
- M. Huang, J. Liu, Y. Li, X.-B. Lan, P. Su, C. Zhao and Z. Ke, Catal. Today, 2021, 370, 114–141 CrossRef CAS.
- A. Prades, E. Peris and M. Albrecht, Organometallics, 2011, 30, 1162–1167 CrossRef CAS.
- C. M. Wong, M. B. Peterson, I. Pernik, R. T. McBurney and B. A. Messerle, Inorg. Chem., 2017, 56, 14682–14687 CrossRef CAS PubMed.
- X. Xie and H. V. Huynh, ACS Catal., 2015, 5, 4143–4151 CrossRef CAS.
- M. Viciano, M. Sanau and E. Peris, Organometallics, 2007, 26, 6050–6054 CrossRef CAS.
- D. Gnanamgari, E. L. O. Sauer, N. D. Schley, C. Butler, C. D. Incarvito and R. H. Crabtree, Organometallics, 2009, 28, 321–325 CrossRef CAS.
- Q. Wang, K. Wu and Z. Yu, Organometallics, 2016, 35, 1251–1256 CrossRef CAS.
- S. Shee, B. Paul, D. Panja, B. C. Roy, K. Chakrabarti, K. Ganguli, A. Das, G. K. Das and S. Kundu, Adv. Synth. Catal., 2017, 359, 3888–3893 CrossRef CAS.
- V. K. Singh, S. N. R. Donthireddy, P. M. Illam and A. Rit, Dalton Trans., 2020, 49, 11958–11970 RSC.
- P. M. Illam, V. K. Singh, Priya and A. Rit, J. Organomet. Chem., 2021, 951, 122008–122016 CrossRef CAS.
- X. Xie and H. V. Huynh, Org. Chem. Front., 2015, 2, 1598–1603 RSC.
- J. Otera, Modern Carbonyl Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed.
- The addition of PPh3 (0.05 mol%) was noted to influence the catalytic activities of complexes 2a/2b. In presence of PPh3, complexes 2a and 2b converted 1-phenylethanol to 5a + 5a′ (with high selectivity towards 5a) in 87% and 98% yields, respectively in 6 h. This enhancement in catalytic activity might be due to the involvement of a different catalytic species.
- C. Schlepphorst, B. Maji and F. Glorius, ACS Catal., 2016, 6, 4184–4188 CrossRef CAS.
- L. K. M. Chan, D. L. Poole, D. Shen, M. P. Healy and T. J. Donohoe, Angew. Chem., Int. Ed., 2014, 53, 761–765 CrossRef CAS.
- X.-N. Cao, X.-M. Wan, F.-L. Yang, K. Li, X.-Q. Hao, T. Shao, X. Zhu and M.-P. Song, J. Org. Chem., 2018, 83, 3657–3668 CrossRef CAS PubMed.
- X. Quan, S. Kerdphon and P. G. Andersson, Chem. – Eur. J., 2015, 21, 3576–3579 CrossRef CAS.
- C. B. Reddy, R. Bharti, S. Kumar and P. Das, ACS Sustainable Chem. Eng., 2017, 5, 9683–9969 CrossRef CAS.
- S. Ogawa and Y. Obora, Chem. Commun., 2014, 50, 2491–2493 RSC.
- L. Bettoni, C. Seck, M. D. Mbaye, S. Gaillard and J.-L. Renaud, Org. Lett., 2019, 21, 3057–3061 CrossRef CAS PubMed.
- A. R. Sahoo, G. Lalitha, V. Murugesh, C. Bruneau, G. V. M. Sharma, S. Suresh and M. Achard, J. Org. Chem., 2017, 82, 10727–10731 CrossRef CAS.
- S. Genç, S. Gülcemal, S. Günnaz, B. Çetinkaya and D. Gülcemal, J. Org. Chem., 2020, 85, 9139–9152 CrossRef.
- F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry, Kluwer Academic, New York, 4th edn, 2001, part B, ch. 3.2.5 Search PubMed.
- Y. Chen, Chem. – Eur. J., 2019, 25, 3405–3439 CrossRef CAS.
- T. T. Dang, B. Ramalingam and A. M. Seayad, ACS Catal., 2015, 5, 4082–4088 CrossRef CAS.
- N. Biswas and D. Srimani, J. Org. Chem., 2021, 86, 10544–10554 CrossRef CAS.
- P. Piehl, R. Amuso, A. Spannenberg, B. Gabriele, H. Neumann and M. Beller, Catal. Sci. Technol., 2021, 11, 2512–2517 RSC.
- P. Liu, N. T. Tung, X. Xu, J. Yang and F. Li, J. Org. Chem., 2021, 86, 2621–2631 CrossRef CAS.
- O. Ogata, H. Nara, M. Fujiwhara, K. Matsumura and Y. Kayaki, Org. Lett., 2018, 20, 3866–3870 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data (NMR, ESI-MS, and crystallographic details) of the synthesized compounds. CCDC 2102241. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cy01767e |
| This journal is © The Royal Society of Chemistry 2022 |