Open Access Article
Open Access ArticlePolymers showing intrinsic antimicrobial activity
Meltem
Haktaniyan
 and
Mark
Bradley
and
Mark
Bradley
 *
*
EaStCHEM School of Chemistry, University of Edinburgh, David Brewster Road, EH9 3FJ, Edinburgh, UK. E-mail: mark.bradley@ed.ac.uk
First published on 27th September 2022
Abstract
Pathogenic microorganisms are considered to a major threat to human health, impinging on multiple sectors including hospitals, dentistry, food storage and packaging, and water contamination. Due to the increasing levels of antimicrobial resistance shown by pathogens, often caused by long-term abuse or overuse of traditional antimicrobial drugs, new approaches and solutions are necessary. In this area, antimicrobial polymers are a viable solution to combat a variety of pathogens in a number of contexts. Indeed, polymers with intrinsic antimicrobial activities have long been an intriguing research area, in part, due to their widespread natural abundance in materials such as chitin, chitosan, carrageen, pectin, and the fact that they can be tethered to surfaces without losing their antimicrobial activities. In addition, since the discovery of the strong antimicrobial activity of some synthetic polymers, much work has focused on revealing the most effective structural elements that give rise to optimal antimicrobial properties. This has often been synthesis targeted, with the generation of either new polymers or the modification of natural antimicrobial polymers with the addition of antimicrobial enhancing modalities such as quaternary ammonium or guanidinium groups. In this review, the growing number of polymers showing intrinsic antimicrobial properties from the past decade are highlighted in terms of synthesis; often based on post-synthesis modification and their utilization. This includes as surface coatings, for example on medical devices, such as intravascular catheters, orthopaedic implants and contact lenses, or directly as antibacterial agents (specifically as eye drops). Surface functionalisation with inherently antimicrobial polymers is highlighted and has been achieved via various techniques, including surface-bound initiators allowing RAFT or ATRP surface-based polymerization, or via physical immobilization such as by layer-by-layer techniques. This article also covers the mechanistic modes of action of intrinsic antimicrobial polymers against bacteria, viruses, or fungi.
Introduction
Contamination by pathogens is a major concern in many areas including, implanted medical devices (from catheters to artificial hips), infection due to surgical tools, dental restoration, food packaging and storage, as well water (probably the biggest global infection risk from pathogens).1 In addition, over the past few decades multi-drug resistant pathogens have become an increasing threat to global healthcare systems, largely because of overuse/abuse of antibiotics. As such, novel approaches to the elimination/control of these pathogens would have an enormous impact on public health. Towards this goal antimicrobial polymers are of particular interest as new classes of agents to detect, mitigate, combat and/or diminish infections caused by bacteria, fungi, viruses or parasites.1–4Antimicrobial polymers
The first synthetic antimicrobial polymers were synthesized by Cornell in 19655 as homo and copolymer derivatives of 2-methacryloxytroponones. The design of these antimicrobial polymers was based on host defence peptides and polymer disinfectants6 and this was followed in the 1980s by the generation of various salicylic acid functionalized polymers which showed antibacterial activity.7 The antimicrobial activity of natural polymers such as chitosan8 and ε-poly-lysine9 were also discovered/recognised. In 1984, Ikeda10 synthesized a number of cationic polymers based on poly vinyl benzyl ammonium chloride and this has been recognised as a key breakthrough in the area of cationic antimicrobial polymers. Since this time research related to antimicrobial polymers has gained momentum and several reviews on antimicrobial polymers have been published.2,11–16Over the past few decades, there have been incredible efforts to find ever more effective polymer-based antimicrobial agents, either by synthesizing new polymers with different structures, compositions, or architectures or by attempting to enhance the antimicrobial action of existing antibacterial polymers by functionalization. Thus, some polymers, which have no antimicrobial action, can be functionalized with specific groups such as guanidium or quaternary ammonium groups and/or combined with silver nanoparticles to generate antimicrobial properties. Existing polymers with innate antimicrobial activities such as chitin, chitosan, agarose or carrageen have long been of interest, since their abundance and multiple functional groups makes them good candidates for enhancement of their inherent (although limited) antimicrobial activity by modification.17–19
In general, bacteria are classified into two groups, Gram-positive or Gram-negative based on their cell envelope structures. For example, Gram-positive bacteria such as S. aureus, E. faecium, S. epidermidis etc. have an inner cytoplasmic membrane covered by a very thick peptidoglycan layer decorated with teichoic acid and lipoteichoic acid. Contrastingly, Gram-negative bacteria2 have an additional outer membrane made up mostly liposaccharides and phospholipids as an upper layer on top of a thinner peptidoglycan layer. M. tuberculosis20 is an extremely important bacillus that causes Tuberculosis, a leading cause of worldwide morbidity and mortality (in excess of 1.5 million deaths per annum) – and the major contributor to deaths caused by multiple drug resistant bacteria. M. tuberculosis is classified as neither Gram-positive nor Gram-negative due to its unique cell envelope composed of three parts: mycolic acid, arabinogalactan polysaccharides and peptidoglycans. As for their antimicrobial mechanism of action, although many papers have tried to explain the working mechanisms of these polymers against pathogens, this phenomenon remains largely a mystery/obscure and clearly varies across bacterial genus. Thus, depending on polymer composition, different antibacterial actions can be observed. To illustrate this, cationic polymers seem mainly to interact with the cell envelope of bacteria that is formally anionic due to the presence of teichoic acid and lipoteichoic acid in the case of Gram-positive bacteria, and liposaccharides and phospholipids in Gram-negative bacteria via electrostatic interactions. This local neutralisation of charge is destabilizing and seems to increase the permeability of the cell membrane leading to bacterial death. The interaction of polymers with M. tuberculosis is typically driven by hydrophobic interactions due to its waxy, lipid-rich, membrane as well as cationic interactions. Enveloped viruses such as SARS-CoV-2, SARS coronavirus, Ebola virus, HIV, influenza virus and so on are also protected by a lipid membrane21 and it is believed that hydrophobic interactions are a key interaction by which antimicrobial polymers can destroy enveloped viruses. Recently polysulfonated polymers have been used to eradicate viral surface contamination, with the coating effective over multiple cycles, with the local “highly acidic” environment believed to be responsible for their mode of action. Thus, the antimicrobial mechanism of polymers differs based on the type of pathogen and the interaction of the polymer with the specific microorganism. Typically, the action of antimicrobial polymers can be categorized as either direct killing (bactericidal/fungicidal or virucidal) by contact and/or inhibition of the growth of the microbes (bacteriostatic) in solution or on surfaces.
This review gives a detailed literature overview (from the last decade) on the current state-of-the-art of polymers which demonstrate an intrinsic antimicrobial mode of action either when immobilised on a surface via covalent or physical attachment, or as a solution formulation, with the synthesis and mechanism of antimicrobial action of the polymers given in detail throughout the review.
1. Polymers immobilized on surfaces
1.1. Polymers physically attached to surfaces
Polymers can be attached to surfaces by a number of physical or chemical interactions and coated by a variety of coating processes such as, spin-coating, dip-coating, solution casting, spray coating and many other generic printing techniques22 that layers polymers onto a substate/surface. Physical attachment of polymers onto a surface is simple and facile and applicable to a variety of surfaces; compared to chemical attachment; with few requirements for complex surface processing. However, uniformity of coating and the stability of the coating due to leaching from the surface should be considered as limitations. Polymers can be coated onto surfaces as a simple binary composition, as a blend of polymers, or as multilayer films.Access to clean drinking water has huge importance to human health however, almost half a billion people across the globe lack access to clean water with contamination by viruses and bacteria a serious threat.25 Sinclair et al.26 modified negatively charged commercial flat sheet polyether sulfone microfiltration membranes with PEI, to create a virus-free water purification system. Based on the electrostatic interactions between the membrane and the cationic polymer, the polymer (molecular weight 25 and 750 kDa) was deposited onto membrane in various ratios (0.3–1.3 wt%). Due to the coating by PEI, the pore size of membrane decreased slightly with ∼22% loss of membrane permeability, however, this simple modification of commercially available membranes led to substantial viral reductions with flow of 5000 L m−2 in approximately 2.5 h.
Nagaraja et al.27 prepared hydrophilic, antimicrobial, thin film surface coatings with maleic anhydride-N-vinylpyrrolidone copolymers which were readily functionalised with aminophenol, generating an amide bond and a free carboxylic acid on the polymer with the addition a phenolic group. These polymers were created by dip coating, with thicknesses of 1.63 μm on glass and 1.75 μm on metal. The antimicrobial action of the coated surfaces was tested against the pathogens S. aureus, E. coli, M. smegmatis and C. albicans and SEM images showed that the polymeric thin film effectively killed all four microorganisms causing disruption of the bacterial and fungal cell membrane with the authors suggesting this was mediated via the phenolic groups of the polymers, presumably mediated via ROS generation.
Cationic polymers are effective materials to fabricate contact-active surface coatings, but in addition, hydrophobic modification of the polycationic materials also enables them to interact/penetrate into hydrophobic bacterial cell membranes leading to bacteria lysis. Based on this concept, Westman et al.28 investigated hydrophobically modified polyvinylamines (a highly charged (pH dependent) polyelectrolyte) to prepare coatings active against E. coli and B. subtilis (Table 1 – P11). Polymers were synthesized by hydrolysis of polyvinylformamide with various degrees of conversion of the amide groups into amines. Some of the amine groups were subsequently derivatised utilizing epoxy-alkanes of various chain lengths (hexyl, octyl, dodecyl), and the polymers were used to prepare antimicrobial coatings on glass slides by physical adsorption. The hexyl-modified polyvinylamines showed the most potent activity against E. coli while the octyl derivative displayed greater activity against B. subtilis.
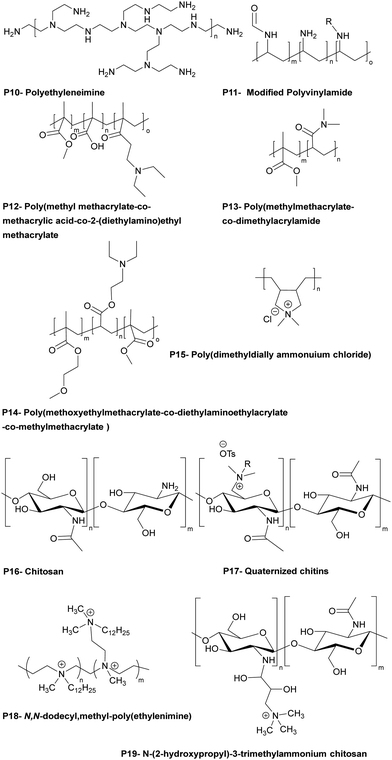
The Bradley group developed a number of polymer microarray platforms for the high-throughput screening and identification of biomaterials and examined pathogen attachment and their interaction with polymeric surfaces. One of their studies29 focused on the identification of novel materials that could rapidly either selectively bind or repel the food-borne pathogens S. typhimurium and E. coli. In this screen 16 polyacrylates based on methyl methacrylate and glycidyl methacrylate (functionalized with different amines) were also found to inhibit S. enterica binding. A polymer composed of methyl methacrylate/methacrylic acid and 2-(diethylamino)ethyl methacrylate, effectively inhibited the adhesion of both S. typhimurium and E. coli (see Table 1 – P12). Interestingly some polyacrylates showed selective binding of E. coli, while not binding S. typhimurium. Among the bacteria binding polymers, S. typhimurium appeared firmly attached on (poly(hydroxyethyl methacrylate-co-dimethyl amino ethyl methacrylate)) coated surfaces. When screening libraries of polyurethanes, polymers synthesised using the diols polybutylene glycol and polypropylene glycol (with a range of diisocyanates) showed selective binding of S. typhimurium. In another study30 interaction of the waterborne protozoan pathogen G. lamblia with polymer modified surfaces were investigated. From the 652 screened polymers, 34 hit polymers were identified and investigated in more detail to understand/generate a structure–property relationship. This showed that amide, glycol, and aromatic containing polymers inhibited the adhesion of the pathogen, whereas amine groups containing polymers promoted adhesion. The same group31 screened a library of polyacrylates/acrylamides, synthesized by free radical polymerisation, to discover anti-adhesive polymeric catheter coatings, looking at the inhibition of binding of mixtures of clinically isolated bacteria (K. pneumoniae, S. saprophyticus and S. aureus or K. pneumoniae, S. mutans, S. aureus, and E. faecalis). Due to their flexibility and coating abilities poly(methyl methacrylate-co-dimethylacrylamide) and poly(methoxy ethylmethacrylate-co-diethylaminoethylacrylate-co-methylmethacrylate) (Table 1 – P13, P14) were chosen for coating of polyurethane-based multi-lumen central intravenous catheters and silicone-based double lumen catheters, with 10% w/v acetone coating solutions showing repelling properties against various microorganisms. On both catheters coating by poly(methylmethacrylate-co-dimethylacrylamide) displayed the best performance, reducing by >96% bacterial binding onto the polyurethane catheter and by >82% onto the silicone catheter.
Hook et al.32 studied polymers using microarray technique showing bacteriostatic (inhibition of growth of bacteria) action using a polymer library produced by mixing 22 acrylate monomers containing ethylene glycol chains of various lengths, fluoro-substituted alkanes, linear and cyclic aliphatic, aromatic and amine moieties. Polymers synthesized by catalytic chain transfer polymerization showed high bacteria adhesion resistance and were coated onto silicone catheters by dip coating after oxygen plasma activation and antimicrobial performance of the coated silicone catheters was compared to commercial silver hydrogel coated latex catheters. Among the hit polymers, a homopolymer of ethylene glycol dicyclopentenyl ether acrylate decreased P. aeruginosa attachment by 28-fold and 17-fold compared to bare silicone catheter and silver hydrogel coated latex catheter. For S. aureus, copolymers consisting of cyclic monomers [([8-(prop-2-enoyloxymethyl)-3-tricyclo[5.2.1.02,6]decanyl]methyl-prop-2-enoate)] and 4-tert-butylcyclohexyl acrylate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio) showed a 67-fold reduction and a 30-fold reduction of bacterial binding compared to bare silicone and silver hydrogel coated latex catheters. Analysis of the structure property relationships from the microarray screens allowed the same group33 to select 116 (meth)acrylate monomers to generate new polymers predicted to resist bacterial attachment. Among the hits, materials were identified that resisted attachment of P. aeruginosa, S. aureus, and uropathogenic E. coli, reducing bacterial coverage up to 81%, 99%, and 99%.
3 ratio) showed a 67-fold reduction and a 30-fold reduction of bacterial binding compared to bare silicone and silver hydrogel coated latex catheters. Analysis of the structure property relationships from the microarray screens allowed the same group33 to select 116 (meth)acrylate monomers to generate new polymers predicted to resist bacterial attachment. Among the hits, materials were identified that resisted attachment of P. aeruginosa, S. aureus, and uropathogenic E. coli, reducing bacterial coverage up to 81%, 99%, and 99%.
Dundas et al.34 developed a quantitative structure–activity relationship of materials synthesized from (meth)acrylate-based polymers in relation to bacterial biofilm resistance. Among the coatings, catheters coated with a polymer generated using the novel monomer (cyclododecyl methacrylate) reduced by 55-fold binding by six urinary tract pathogens compared with silicone catheters and 14-fold compared to silver hydrogel coated catheters. In this area, Adlington et al.35 used the monomer, (di(ethylene glycol)) methyl ether methacrylate, to provide elasticity, while decreasing the of Tg value of the copolymers. It was also found that a copolymer synthesized from the monomers: ethylene glycol dicyclopentenyl ether acrylate and diethylene glycol methyl ether methacrylate (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) decreased by 25-fold bacterial attachment of P. aeruginosa, S. aureus, E. coli compared to neat silicone and silver hydrogel coated catheters.
25) decreased by 25-fold bacterial attachment of P. aeruginosa, S. aureus, E. coli compared to neat silicone and silver hydrogel coated catheters.
So-called “self-sterilizing surfaces” are a growing area. Bharadwaja et al.36 developed a self-organizing, amphiphilic (anionic) material, made of multiblock polymers (with the mid-block selectively sulfonated) that showed antibacterial and virucidal activity. Two commercially available poly[tert-butylstyrene-b-(ethylene-alt-propylene)-b-(styrenesulfonate)-b-(ethylene-alt-propylene)-b-tert-butyl styrene] pentablock polymers (with mid-block degrees of sulfonation of either 26 mol% or 52 mol%) spontaneously self-assemble into nanostructures and were evaluated against Gram-negative and Gram-positive bacteria. Polymeric films were prepared and, due to their architecture, permitted water-induced swelling/hydrogel formation. Both polymeric films showed a capability to inactivate S. aureus (99.99%) (including methicillin resistant) within 5 min and showed similar levels of inactivation of E. faecium and three Gram-negative bacteria (A. baumannii, K. pneumoniae and E. coli). Moreover, these polymers inactivated vesicular stomatitis and influenza A viruses within 5 min with a minimum inactivation level of 67 Plaque forming units mL−1. To explore the role of the mid-block sulfonation, a complementary triblock polymer poly(tert-butyl styrene-b-styrene-b-tert-butylstyrene) was synthesized by living anionic polymerization, and mid-block sulfonated (17, 40 and 63 mol%). Testing on S. aureus revealed that between 26% and 40% sulfonated groups were required to eliminate 99.99% of bacteria. Coronavirus 2 (SARS-CoV-2) can be easily transferred via different routes including surface contact and airborne droplets.37,38 After the discovery of the excellent antimicrobial and antiviral activities of the sulfonated polymers, the same group4 tested sulfonate group bearing polymers (poly[tert-butyl styrene-b-(ethylene-alt-propylene)-b-(styrene-co-styrenesulfonate)-b-(ethylene-alt-propylene)-tert-butyl styrene]), poly[tert-butyl styrene-b-(styrene-co-styrene sulfonate)-b-tert-butyl styrene] and (poly[(styrene-co-styrene sulfonate)-b-(ethylene-co-butylene)-b-[(styrene-co-styrenesulfonate)]]) for inactivation of SARS-CoV-2. The ordered lamellar morphology of the polymer surfaces prepared with >50 mol% of sulfonate groups (e.g. poly[tert-butylstyrene-b-(ethylene-alt-propylene)-b-(styrene-co-styrene sulfonate)-b-(ethylene-alt-propylene)-tert-butyl styrene]) were the most effective in the deactivation of HCoV-229E (<5 min). The mechanism of these polymers is believed to be due to the dramatic pH drop at the polymer/pathogen interface (dependant on the number of sulfonate acid groups on the polymer chain), with rapid pathogen inactivation observed.
Keum et al.39 develop trifunctional antimicrobial, antiviral, and antibiofouling polymers that could be readily coated onto the surface of medical protective clothing. The coating polymers were synthesized by free radical polymerization using various ratios of the monomers (lauryl methacrylate), poly(ethylene glycol) methacrylate and 2-(dimethylamino)ethyl methacrylate quaternized with methyl iodide. Regardless of the monomer ratios explored, all polymers reduced S. aureus by >75% and reduced bacterial adhesion by >65%. When sprayed the polymer gave a nano coating layer on hydrophobic surfaces of personal protection equipment, with the spray coated polymer surfaces remaining stable for 24 hours and maintaining their antiadhesion and bactericidal activities. Interestingly, fabric surfaces coated with polymers with lower levels of the quaternary ammonium groups, showed better deactivation activity of porcine epidemic diarrhoea virus (a coronavirus that bears a structural resemblance to the prevailing SARS-CoV-2), reducing virus viability on the surface, compared with the uncoated surface and higher content quaternary ammonium units bearing polymers. The most effective polymers were biocompatible with mammalian cells compared to bacteria and viruses and showed no recognizable local or systemic inflammatory responses in animal experiments.
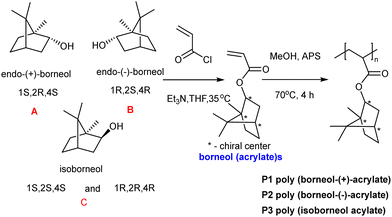
Mussel inspired dopamine-based materials have gained interest due to their adhesive capabilities and their applicability in biomedical applications as antifouling materials,40 self-healing materials,41 their use in separations,42 and in cell and tissue engineering.43 Wang et al.44 designed, and synthesized, by RAFT polymerization, a durable, adhesive, antimicrobial coating, based on the diblock poly[(N-3,4-dihydroxyphenethyl acrylamide)-b-(borneol acrylate)]. Here, the bio-based monomers were synthesized from dopamine that provides the polymers its adhesive properties (as well as phenols for reactive oxygen generation) and borneol which is natural antibiotic found in “medical” herbs such as lavender, valerian, and chamomile. Prior to polymerization dopamine acrylate was protected with triethylsilane groups and then polymerized with the chain transfer agent, 2-cyanomethyl-s-dodecyl trithiocarbonate, to generate a macro raft agent (PDA10, Mn: 4.7 kDa, PDI: 1.03). This was used to synthesize copolymers with various block ratios and among the synthesized copolymers, PDA10-b-PBA55 was selected to perform adhesion and antibacterial tests and was coated onto various substrates. The coated surfaces showed high integrity after rinsing with both water or chloroform and after a peel-off-test due to attachment by the 3,4-dihydroxyphenyl groups. The polymer (PDA10-b-PBA55) when coated onto stainless-steel surface inhibited the growth of bacteria (up to 93%) for E. coli and 83% for S. aureus (Fig. 1 and 2).
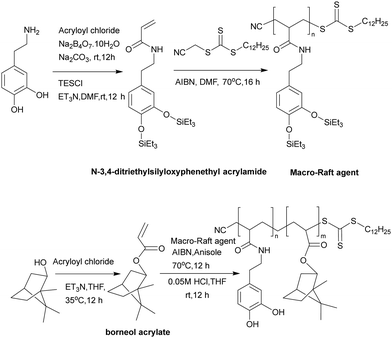 | ||
| Fig. 1 Synthesis of the monomers: N-3,4-dihydroxyphenethyl acrylamide and borneol acrylate and the synthesis of poly[(N-3,4-dihydroxyphenethyl acrylamide)-b-(borneol acrylate)] via RAFT polymerization.44 Reproduced from ref. 44 with permission from Elsevier. | ||
 | ||
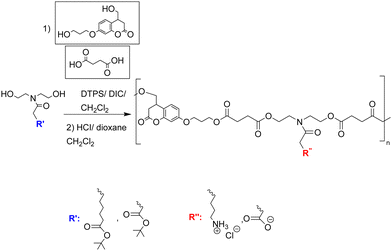
| Fig. 2 The synthesis of poly(borneol acrylate)s from borneol (acrylate)s.45 Reprinted (adapted) with permission from {L. Luo, G. Li, D. Luan, Q. Yuan, Y. Wei and X. Wang, Appl. Mater., 2014, 6, 19371–19377}. Copyright {2014} American Chemical Society. | ||
The isomers of borneol acrylate were used to prepare a series of borneol-based polymers to understand antibacterial adhesion in relation to monomer/polymer stereochemistry with polymers coated onto Si-based substrates (approximately 10 μm thickness). Borneol acrylate-based polymer films and the control poly(methyl methacrylate) were cut into circular rings. E. coli were placed into the rings and incubated with for 60 h. The poly(borneol acrylate) film rings acted as “perfect prisons”, with negligible amounts of E. coli observed on the inner and outer surfaces of the ring compared to the control. Among the poly(borneol acrylate) polymers, poly(borneol-(+)-acrylate) and poly(isoborneol acrylate) showed similar activity against E. coli, S. aureus and M. racemosus by inhibiting the bacteria growth on the surface. However, D configurated borneol polymer showed weak antimicrobial action compared to other polymers. Poly(borneol acrylate)s caused the lysis of bacteria (clearly seen in SEM) images in which hydrophobic–hydrophobic interactions between the polymer and bacteria cause the loss of integrity of bacteria after being exposed to poly(borneol acrylate) films. Here, they showed that surface stereochemistry, and especially the bicyclic structure, is crucial for poly(borneol acrylate)s antibacterial adhesion.45
The antifungal activity of poly(borneol acrylate)s was explored by Xu et al.46 who developed a non-toxic, antifungal coating to treat fungal biodeterioration in paper production. Poly(borneol acrylate)s were synthesized in methanol with ammonium persulfate as an initiator at 70 °C and coated onto paper after dissolving in DCM and spray coating. The antifungal activity of the “polymer paper” was tested against A. niger and Penicillium sp., fungi that can easily colonize the surfaces of most materials and rapidly spread via fungal spores. Therefore, inhibition to stop the spread of fungal spores is key to creating antifungal coatings. After incubating the polymer coated papers with fungi over eight days, only scattered spores were found on the 10 and 15% poly(borneol acrylate) coated papers. In addition, SEM images found almost no sporangia or hypha and only a few scattered spores could be found on the surface. There is no simple explanation to explain the antifungal mechanism, probably due to hydrophobic nature of the polymer the fungi did not adhere to the surface and prevented the spread of fungal spores on the surface.
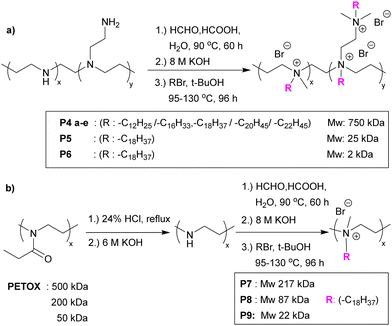
Hospital-acquired infections are one of the biggest threats/risks to humans who enter hospitals, due to the long-term use/abuse of antibacterials and other chemicals (such as anticancer agents) within that environment that drive resistance. As such patients are exposed to antibiotic resistant bacteria in routine hospital procedures. P. aeruginosa is commonly found to be resistant to antibiotics and can cause serious infections including ventilator-associated pneumonia, catheter associated sepsis and wound-burn infections.25 To understand which pendant group shows the best activity against P. aeruginosa, Chamsaz et al.47 developed a coumarin containing polyester coating containing amine or carboxylic acid pendant chains with coumarin and aliphatic diols, and succinic acid making up the main backbone. The monomers (see Fig. 3) with t-butyl protected carboxylate acid groups (giving the anionic pendant groups upon deprotection) or Boc-protected amine group (giving the cationic pendant groups) were polymerized by carbodiimide-mediated polymerization of the diols and diacids with the films prepared by spin coating and then deprotected. Only the polymers carrying cationic pendant groups killed bacteria attempting to attach to the surface and prevented biofilm formation. When the polymer film was crosslinked (via irradiation, with the coumarin units undergo a [2+2] photocycloaddition reaction to form crosslinked coatings) similar results against P. aeruginosa were observed, without any leaching of oligomeric species. As before, the antimicrobial activity is based on the amino groups of the polymers interacting with negatively charged bacterial components. The most active polymer showed no cytotoxicity or hemolytic activity.
 | ||
| Fig. 3 Synthesis route to coumarin containing polyesters with pendent amine or carboxylic acid groups.47 Reprinted (adapted) with permission from {E. A. Chamsaz, S. Mankoci, H. A. Barton and A. Joy, Appl. Mater., 2017, 9, 6704–6711}. Copyright {2017} American Chemical Society. | ||
 | ||
| Fig. 4 (a) Functionalization of branched PEI via Eschweiler–Clarke methylation and quaternization with various alkyl bromides. (b) Linear PEI polymers synthesised from poly(2-ethyl-2-oxazoline) and modified via Eschweiler–Clarke methylation and quaternization with alkyl bromides.48 Reprinted (adapted) with permission from {J. Hoque, P. Akkapeddi, V. Yadav, G. B. Manjunath, D. S. S. M. Uppu, M. M. Konai, V. Yarlagadda, K. Sanyal and J. Haldar, ACS Appl. Mater. Interfaces, 2015, 7, 1804–1815}. Copyright {2015} American Chemical Society. | ||
The same group49 reported the antimicrobial activity of biodegradable chitin coatings that were prepared with various degrees of quaternization of chitin (degree of acetylation ∼75%). Three different “tosyl-chitins” were prepared by reaction with tosyl chloride in a solvent system consisting of LiCl and N,N-dimethylacetamide which allowed selective tosylation of only the least sterically hindered C6-hydroxyl groups. The free primary amine groups were then N-acetylated before substitution chemistry with N,N-dimethyl dodecyl amine, N,N-dimethyl tetradecyl amine, and N,N-dimethyl hexadecyl amine to give quaternized chitin derivatives. The functionalized chitins were spin-coated onto surfaces either alone or blended with polylactic acid. The polymer coated surfaces showed different antimicrobial activities based on the degree of quaternization and alkyl chain length. Amongst all the polymers, polymers quaternized with a C-16 alkyl chain with degrees of quaternization of 39–48% showed profound antimicrobial activities. The minimum inhibitory amount of the surfaces coated with the two most potent polymers were found to be 0.32 μg mm−2 and 0.12 μg mm−2 for methicillin resistant S. aureus, 0.12 μg mm−2 and 0.12 μg mm−2 for against E. faecium, and 15.6 μg mm−2 and 7.8 μg mm−2 against K. pneumoniae. Dip-coated catheters (48% quaternized with C-16 alkyl chains) gave a reduction in bacteria count dependant on the level of coating, with catheters coated at 7.5 μg mm−2 showing a 3.7-log CFU decrease in methicillin-resistant S. aureus, with a negligible number of bacteria binding and no biofilm formation on the surface.
Quaternary ammonium salts of chitosan can show antifungal activity, with the cationic groups of chitosan interacting with negatively charged units within the cell wall of fungi, causing the release of intercellular components. Tabriz et al.50 investigated the antifungal properties of a trimethyl quaternized chitosan, blended with polyether sulfones at various concentration (5%, 10% and 15% w/w) to give membrane films for water treatment applications. The antifungal activity of the polymers was tested with the chitosan blend showing better inhibition compared to quaternized chitosan for F. solani, whereas the trimethyl chitosan was better for A. niger. Additionally, the membrane prepared with the highest concentration of trimethyl chitosan showed a reduction in the number of spores of A. niger and F. solani by 73% and 63%, respectively.
Polyelectrolyte-surfactant complexes have been explored in applications ranging from medicine51 to food and cosmetics.52 Yu et al.53 prepared a water-insoluble catheter coating using electrostatic interactions between poly-L-lysine and the anionic surfactant, 1,4-bis(2-ethylhexyl) sodium sulfosuccinate. The polymer–surfactant complex (in ethanol) adsorbed onto the hydrophobic surface of a polyurethane thermoplastic via hydrophobic interactions to generate a stable coating. Contact-killing antibacterial activity was visualized using SEM and showed lesions and distortions on the cell membrane of S. aureus and E. coli (Fig. 5). In addition, complex coated catheters totally inhibited the adhesion of bacteria under both static and flow conditions, while maintaining antibacterial activity for more than 30 days. An implant-associated bacterial infection experiment showed that poly-L-lysine/1,4-bis(2-ethylhexyl) sodium sulfosuccinate complex coated catheters exhibited excellent inhibition of the inflammatory response, presumably related to the highly acidic succinate surface.4
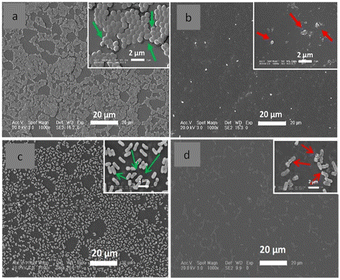 | ||
| Fig. 5 Catheters (coated with poly-L-lysine/1,4-bis(2-ethylhexyl) sodium sulfosuccinate and uncoated) were incubated with S. aureus or E. coli (106 mL−1) for 24 h. SEM images of: (a). images of S. aureus and (c) E. coli binding onto the uncoated catheter; (b) images of S. aureus and (d) E. coli binding onto the complex coated catheters. The green arrows show undamaged bacteria on the uncoated catheter, while the red arrows show the few attached bacteria on the coated catheter with lesions and distortions on the bacterial surface.53 Modified from ref. 53 with permission from Elsevier. | ||
Poly(lactic acid) is one of the most widely used building blocks in the development of 3D printed scaffold/implants due to its ease of printing. However, poly(lactic acid) based substrates lack suitable functional groups which can modified to allow the attachment of polymer brushes directly onto their surface. Thus, Dhingra et al.54 developed a biodegradable 3D-PLA scaffold by blending poly(lactic acid) with tartaric acid based aliphatic polyesters in which the bactericidal polymer, (poly(2-[(methacryloyloxy)ethyl]trimethylammonium chloride) or the antiadhesive polymers ((poly(ethylene glycol) methacrylate)) and poly(2-hydroxyethyl methacrylate)) brushes were tethered. Initially tartaric acid-based biodegradable aliphatic polyesters were synthesized using hexamethylene 2,3-O-isopropylidenetartarate with removal of the isopropylidene groups creating free hydroxyl groups on the polyester. After coating of the polyester onto the glass surface, the hydroxyl groups of the polyester were conjugated to an ATRP initiating moiety which enabled graft polymerization of monomers (2-hydroxyethyl methacrylate,2-(methacryloyloxy)ethyl trimethylammonium chloride and poly(ethylene glycol) methacrylate) from the polymer backbone. The polyester modified with cationic bactericidal 2-(methacryloyloxy)ethyl trimethylammonium chloride brushes showed the highest antibacterial activity (>97% and >96% killing efficiency for E. coli and S. aureus, respectively) and acceptable cytocompatibility. This result showed that the addition of the tartaric acid-based polyesters to PLA could be applicable in the area of biomedical implants.
Poly(ε-caprolactone) is commonly used due to its biocompatibility, non-toxicity, and hydrophobicity; however, poly(ε-caprolactone) has poor mechanical properties and weak cellular affinity. Thus, this polymer was blended with hydrophilic polymers for use in tissue engineering. Aynali et al.55 synthesized biodegradable PLA copolymers by the ring-opening polymerization of L-lactide and cyclic carbonate monomers bearing an azido group (2,2-bis(azidomethyl)trimethylene carbonate) in the presence of 1-dodecanol as an initiator (and tin(II) 2-ethylhexanoate as the catalyst). These polymers were modified with a quaternary ammonium salt (N,N-dimethyl-N-prop-2-yn-1-yldodecane-1-ammonium bromide via “click” chemistry) along its backbone (Fig. 6). The best antimicrobial activity being seen with 30% levels of modification. The same author56 prepared antimicrobial, hydrophilic nanofiber biomaterials via electrospinning with poly(ε-caprolactone) and the previously synthesized PLA copolymer (1, 5 and 8%). Fibres mats were collected and their antimicrobial properties evaluated. It was found that increasing the content of the PLA copolymer in the blend enhanced the antimicrobial activity of the fibres (unmodified poly(ε-caprolactone) showed no antimicrobial activity) with the best blend killing 99.5% and 92% of S. aureus and E. coli, respectively. Clearly, the bactericidal activity of blends is based on the quaternary ammonium groups of the PLA copolymer and functions as a contact active antimicrobial agent (Fig. 7 and 8).
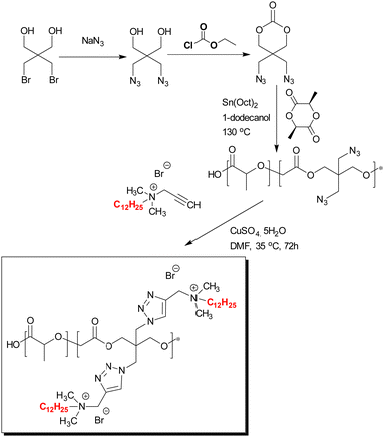 | ||
| Fig. 6 Synthesis of quaternized ammonium group modified PLA-based copolymers.55 Modified from ref. 55 with permission from Wiley. | ||
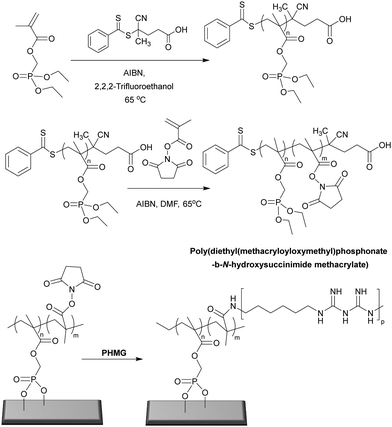 | ||
| Fig. 8 Synthesis of poly(diethyl(methacryloyloxymethyl)phosphonate-b-N-hydroxysuccinimide methacrylate) via RAFT polymerization and the post-modification of poly((methacryloyloxymethyl)phosphonate-b-N-hydroxysuccinimide methacrylate) brush surface with poly(hexamethylene biguanide).97 Reproduced from ref. 97 with permission from Elsevier. | ||
Poly(vinyl alcohol) has been widely used in wound dressing applications due to its hydrophilicity, biocompatibility, and good film-forming abilities. However, it shows poor antimicrobial activity and losses its mechanical properties when wet. Thus, this polymer is typically mixed with others. In this context, Vargoez-Catzim57 blended various concentrations of poly(2-acrylamido-2-methyl-1-propanesulfonic acid) and cross-linked these with succinic anhydride to develop a new wound dressing. By blending poly(vinyl alcohol) with different ratios (5, 10 and 15%) of poly(2-acrylamido-2-methyl-1-propanesulfonic acid) “bilayer membranes” were obtained by the phase inversion method. PVA alone has an MIC value, against S. aureus and E. coli, of 33 mg mL−1 without any bactericidal effect, whereas poly(2-acrylamido-2-methyl-1-propanesulfonic acid)/PVA had significantly lower MIC values (S. aureus 1.36 μg mL−1 and E. coli 39 μg mL−1 – some 1000-fold more active). The sulfonic acid pendant groups giving rise to the bactericidal action against both bacteria is due to the creation of a strongly acidic environment that leads to cell membrane destruction. Importantly, the addition of poly(2-acrylamido-2-methyl-1-propanesulfonic) not only provided antimicrobial activity, but the properties of the film were improved, increasing human cell proliferation and cell viability by enhancing surface porosity as well as biocompatibility and improving water uptake.
Poly(vinylidene fluoride) and copolymers of this polymer are of interest due to their biocompatibility, thermal stability, chemical resistance, and mechanical robustness.58 Han et al.59 prepared poly(vinylidene fluoride-co-chlorotrifluoroethylene) to which were grafted quaternary ammonium groups (trimethylamino ethyl methacrylate) or quaternized (4-vinyl pyridines) via chlorine-initiated atom transfer radical polymerization. The polymers obtained were blended with poly(vinylidene fluoride-co-chlorotrifluoroethylene) and, via solvent casting, used to form free standing films. 5 wt% of the polymer bearing the quaternary pyridinium groups showed an antimicrobial effect of greater than 99% against E. coli, S. aureus, and C. albicans, while all blend films displayed excellent biocompatibility. The bactericidal mechanism of the blends was due to the cationic moieties of the polymers interacting with bacteria via electrostatic interactions resulting in the disruption of the cell wall/membrane.
Bacterial biofilm formation causes complicated and chronic infections. Biofilms are defined as organized bacteria communities embedded in an extracellular polymeric matrix attached to living or abiotic surfaces. The vast majority of hospital-based chronic infections are due to biofilms which can form life-threatening colonizers on biomedical devices such as catheters (central venous, urinary), prosthetic heart valves, and orthopaedic devices. Biofilm formation starts with colonization onto an either abiotic or biotic surfaces by adhesion of bacteria with the help of flagella and pili in Gram-negative bacteria or surface proteins in the case of Gram-positive bacteria. After attachment, proliferation of biofilms is triggered and results in the production of an extracellular matrix composed of exopolysaccharides, protein, DNA, bacteriolytic products, and compounds from the host. The last stage of biofilm formation is colonization and after the first layer of the surface is covered with bacteria the process evolves to generate macro-colonies on surfaces.60 Within this formation bacteria are highly resistant to antibiotic therapy, due to the exopolysaccharide matrix that serves as an anchorage/support matrix and makes them less susceptible to the therapeutic agents.61 In this content, Vishwakarma et al.62 prepared water-soluble synthetic peptidomimetic polyurethanes that were able to disrupt surface established biofilms of P. aeruginosa, S. aureus, and E. coli. The polyurethanes (9–10 kDa) were synthesized by polymerization of hexamethylene diisocyanate and N-functionalized diethanolamide as monomers (via amidation of methyl esters of lysine, alanine, or phenylalanine with diethanolamine). Polymers were synthesized (ratio of 60/40 co-monomers) to tune charge/hydrophobic ratio to mimic the structural peptides while ensuring polymer solubility. To mimic arginine, the synthesized polymers were guanylated post synthesis. The arginine modified polyurethane showed the best broad-spectrum activity with a minimum inhibition concentration of 2–4 μg mL−1 for both Gram-positive and Gram-negative bacteria. On the other hand, all polymer compositions showed broad-spectrum biofilm inhibition at low concentrations (2–16 μg mL−1) for P. aeruginosa, S. aureus and E. coli. The polymers were also tested under flow conditions, with biofilms of P. aeruginosa grown for 7 days with or without the polymer. In the presence of the Lys/Phe bearing polymers only surface-attached bacteria were observed (no biofilms). An outer membrane permeability assay showed that these polymers did not exhibit any significant membrane permeabilization and that the polymers inhibit the biofilm formation without direct killing of the bacteria via membrane destruction.
1.1.3.1. Bactericidal PEMs. Some PEMs are bactericidal with contact mediated killing of microorganisms, often driven by the deposition of a cationic polymer top layer which drives the interaction between the surface and the bacteria with disruption of the outer membrane of Gram negative bacteria.67 Wulandari et al.68 produced a silk porous sponge coated via layer-by-layer with the cationic antimicrobial polymer poly((dimethyl-hexyl ammonium bromide) ethyl methacrylate) that was synthesized by free radical polymerization and used with anionic sodium alginate for LbL generation via electrostatic interactions. 5-Bilayer films with an outermost layer of the cationic polymer were sufficient for maximal antimicrobial activity (4-log reduction) against Gram-positive and Gram-negative bacteria. It is worth noting that the coating was non-leachable, and the sponge could be reused up to three times after washing. Furthermore, the same polymers were coated (using the same conditions) onto other substrates, including cotton-based bandages and gauzes and these expressed antimicrobial activities similar to the silk sponge. The antimicrobial action of the coated sponge is based on the top layer coating of the cationic polymer, whose quaternary ammonium centre enables binding with the anionic bacteria, while the hydrophobic n-hexyl tail facilitates insertion into the bacteria membrane.
Polyethyleneimine is a synthetic, aliphatic and polycationic polymer (due to the presence of protonated primary, secondary and tertiary amino groups) which also enables interaction with polyanions.69 The bactericidal action of PEI is driven by the cationic groups which bind to cell membrane phospholipids via electrostatic interactions and en masse lead to rapture.70 However additional positive charge density and the addition of hydrophobic alkyl groups improved the bactericidal activity of the modified PEIs (see the section on blended films). Wong et al.71 showed that linear N,N-dodecyl-methyl-poly(ethylenimine) (Table 1 – P18) and poly(acrylic acid) PEMS exhibited potent bactericidal activity against S. aureus and E. coli as well as antiviral activity against influenza A/WSN (H1N1). N-Alkylated PEIs were synthesized by acid hydrolysis of commercial poly(2-ethyl-2-oxazoline) and alkylated with various bromo alkanes and then quaternerised with iodomethane to produce linear N,N-dialkyl-N-methyl-poly(ethylenimine)s. Polymers were deposited onto silicone substrates alternating with poly(acrylic acid) with the last coating layer of the multilayers being polycationic. The microbicidal action of the films was explained by the initial electrostatic interactions between the cationic surface and the negatively charged bacterial cell wall/membrane and subsequent diffusion of the hydrophobic alkyl chain through the lipid bilayer, leading to disruption of the bacterial membrane. The bactericidal activities of the multilayer films were altered in accordance with the length of the hydrophobic alkyl chain of the cationic polymers and the positive charge density on the film surface which were influenced by the deposition pH of the polyacrylic acid. For example, at low pH (pH 3), poly(acrylic acid) is weakly polyanionic and has a loop conformation in solution. When functionalized PEI polymer chains are adsorbed onto the polyacrylic acid, a thick film results with a “loopier”, more brush-like architecture which enables greater positively charge on the film surface to interact with the bacterial membrane (Gram-negative). Arguments were made that upon increasing the pH to higher values (pH 7), the poly(acrylic acid) chains undergo a conformational change forming a thin random coil conformation with many more negative charges, and that these polymer chains showed greater electrostatic interactions with the functionalized PEI leading to “shielding” of the interaction of positive charges of PEI with the bacterial cell membrane, resulting in the decrease in antibacterial activity of the films. In connection with hydrophobicity, it was observed that the polymer P18 (N,N-dodecyl-N-methyl-poly(ethylenimine), with the longest alkyl side chains) showed the best bactericidal activity against S. aureus and E. coli. To achieve 100% bactericidal and 60% antiviral performance a LbL films composed of a (PEI/poly(acrylic acid))15 bilayer with PEI on top, with poly(acrylic acid) deposition at pH 3 was found to be optimal.
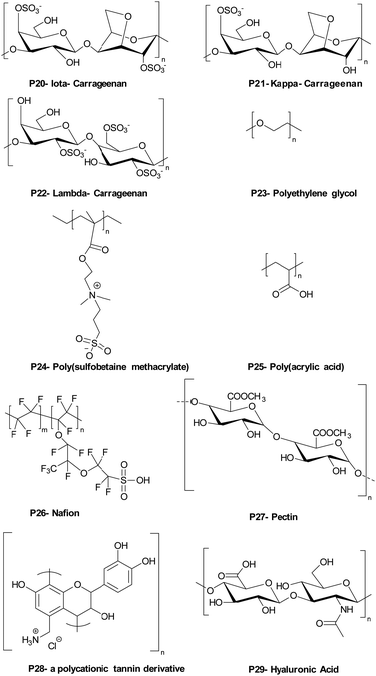
Carrageenans are natural, biocompatible, biodegradable sulfated polysaccharides, with three major classes which vary in the number and position of the sulphate groups on the galactose unit. They are excellent film forming materials. Following on from previously studies, where κ-carrageenan oligosaccharides showed antimicrobial activities against E. coli, S. aureus, S. cerevisiae, P. citrinum and Mucor. sp.,72 Briones et al.73 prepared contact-killing surfaces from PEI and κ-carrageenan (kappa), ι-carrageenan (iota), λ-carrageenan (lambda) (Table 2: P20, P21, P22) via a layer-by-layer self-assembly driven by the electrostatic interactions between positively charged PEI and the negatively charged sulfate groups of carrageenan (6 layers of coating). The results showed that PEI displayed no inhibition of bacterial growth for S. aureus and E. cloaceae and 76% inhibition growth for E. faecalis. λ-Carrageenan inhibited S. aureus by some 25%, while ι-carrageenan inhibited E. faecalis by 48% and E. cloaceae by 40%. The highest inhibition against S. aureus and E. cloaceae was obtained by PEI/iota carrageen (67% and 85% reduction), while the greatest inhibition against E. faecalis was 78% for PEI/lambda carrageenan. The results revealed that there was a synergetic antibacterial effect when PEI and carrageen were used as multilayer coatings, while again the power of poly-sulfonated materials is highlighted.
The well-known natural polycation chitosan has inherent antimicrobial activity against many pathogens, with three modes of action: lysis of the pathogens membrane, blocking bacteria from access to nutrients due to its strong metal-chelating properties as well as its DNA-binding ability.74 Many layer-by-layer assemblies of antibacterial surface coatings based on chitosan have been formed using both synthetic and natural polymers. Kumorek et al.75 constructed films using quaternized (trimethylammonium) functionalized chitosan(N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride) (see Table 1 – P19) and chitosan with tannic acid (TA) to evaluate their antibacterial activities against E. coli and S. aureus. Multilayers were deposited either on quartz slides with chitosan (see Table 1 – P16), N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride or TA-terminated layers – with 9 or 10 layers deposited. Contact-killing efficiency of the films showed a dependency on the terminal polymer layer coating and chitosan type. Thus, chitosan and N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride terminated LbL films showed better antibacterial activity than the TA-terminated films. Furthermore, N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride terminated multilayers showed improved inhibition of initial bacteria attachment, while the chitosan terminated multilayers were three times more effective at killing bacteria.
Hernández-Montelongo et al.76 prepared LbL films of hyaluronan/chitosan and investigated their physicochemical properties and potential as antimicrobial materials. A 9-mer LbL film was prepared from hyaluronic acid and chitosan with the top layer being chitosan. Films were deposited onto silicon wafers as gel-type films at different pH's (pH 3.0 and pH 4.5) and ionic strengths to see how these changes altered the properties of the multilayer films and affected their antibacterial activity. The addition of salt into the deposition solutions affected the swelling behaviour of the PEM leading to the production of thicker films, while the pH of the deposited film solutions affected the interaction between polymer layers and the antibacterial activity of the films. To illustrate, at pH 4.5, hyaluronic acid (pKa: 3.0) and chitosan (pKa: 6.5) were largely in their ionized forms, and the polymer layers electrostatically interacted with each other strongly, thus blocking or shielding of the ammonium group of chitosan by the carboxylic groups of hyaluronic acid. However, at pH 3.0, many of the carboxylic acid groups on hyaluronic acid are non-ionised resulting in reduced interactions with the chitosan and allowing a higher number of ammonium groups to be exposed on the surface to interact with bacterial membranes. Thus, films of chitosan/hyaluronic acid constructed at pH 3.0 showed better bactericidal action against X. fastidious a plant pathogenic bacterium (that causes diseases of important crops) than films prepared at pH 4.5.
Another important antimicrobial polymer is ε-poly-L-lysine (ε-PPL), indeed polymers having 25–35 L-lysine residues is widely used in many countries as a food preservative. Zhang et al.77 prepared ε-poly-L-lysine and gum Arabic multilayer films deposited on anodized titanium with the help of polydopamine with the ε-poly-L-lysine immobilized onto the surface in various ways. Thus, titanium surfaces were anodized in the presence of 0.5 wt% hydrofluoric acid to generate titanium dioxide nanotubes, which were immersed into dopamine hydrochloride to coat the surface with polydopamine via self-polymerization under slightly alkaline conditions (2 mg mL−1 dopamine in 10 mM Tris-HCl, pH = 8.5). Later, the first layer of ε-poly-L-lysine (10 mg mL−1) was covalently grafted onto the polydopamine surface via reaction between the amines and the dopamine quinone (under alkaline conditions) and later gum Arabic was deposited onto the ε-PLL via electrostatic interactions. LbL films were also deposited onto the titanium dioxide nanotube surface by immersing into ε-PLL and gum Arabic. One-layer of ε-poly-L-lysine grafted onto the surface displayed better bactericidal activity than the dual ε-poly-L-lysine-gum surface, showing rupturing of the cell membranes of S. aureus and E. coli. The triple-layer film (ε-PPL/GA/ε-PPL) and the penta-layer film (ε-PPL/GA/ε-PPL/GA/ε-PPL) inhibited the attachment of bacteria onto the surfaces. Furthermore, the layer-by-layer coated surfaces maintained their bacteriostatic action against S. aureus and E. coli for up to 3 weeks, again the cationic nature of the polymer driving their mode of action.
As mentioned previously, polymers that are cationic and hydrophobically modified show potent contact-killing activity against bacteria. Hydrophobically modified polyvinylamines are an interesting class of polycations which possess a high ratio of cationic groups and alkyl chains that form antibacterial surfaces (see the single polymer coating section). Thus, Illergård et al.78 used hydrophobically modified polyvinylamines to prepare multilayer films with poly(acrylic acid) for an antibacterial surface coating. Multilayers were prepared by immersing, alternatingly, polyvinylamine at pH 7.5 as the cationic polymer and poly(acrylic acid) at pH 3.5 as the anionic polymer. Polymers were coated onto the surface with a number of different layers with the polycationic as the top layer. The 3-layer coated surface prepared with C8-epoxy chain modified polyvinylamine showed growth inhibition of >99%. A live/dead assay proved that the bactericidal activity of the films, damaged the bacterial membrane. Later, they79 coated anionic cellulosic wood-fibre with C8-epoxy chain modified polyvinylamine (100 mM NaCl, pH 9.5) and poly(acrylic acid) (100 mM NaCl, pH 3.5). Fibres were coated to give one, three and five layered materials (always with a polyvinylamine outmost layer). The films reduced the growth of E. coli and B. subtilis by 99% after 24 h of contact. The results revealed that even a simple layer coating was sufficient to generate a contact-active surface. To gain insight into the antibacterial mechanisms of physically adsorbed multilayers of polyvinylamine and polyacrylic acid, the surface charge of cellulose fibres was increased via radical oxidation (giving higher concentrations of carboxyl groups on the surface) leading to enhanced immobilization levels of polyvinylamine onto the fibre. In this case, the bactericidal action against E. coli and B. subtilis increased, while SEM images showed that the contact-killing activity of the fibre was due to membrane deformation.80
Chen et al.81 investigated cellulose fibre LbL coatings with different polycations – namely poly(diallyl dimethyl ammonium chloride) (Table 1 – P15) and poly(allylamine hydrochloride). Results showed that a triple-layer poly(vinylamine)/poly(acrylic acid)/poly(vinylamine) showed better antibacterial activity compared to poly(diallyl dimethyl ammonium chloride)/poly(acrylic acid) and poly(allylamine hydrochloride)/poly(acrylic acid). The same group82 changed the middle section (poly(acrylic acid)) to a wood-based cellulose nano-fibril, in order to enhance the antibacterial efficacy of the poly(vinylamine) based films. Bacteria adhesion increased on the fabricated of a triple-layer poly(vinylamine)/cellulose nano-fibril/poly(vinylamine) system but was some 10-fold better compared to the poly(acrylic acid)/poly(vinylamine) film in terms of membrane damage with elongation of the bacteria clearly seen.
1.1.3.2. Bacteriostatic PEMs. An alternative method to tackle pathogen colonisation is the generation of microbe-adhesion resistant surfaces, often by making the surface more hydrophilic. As an example, polyethylene glycol has been widely investigated as an antifouling surface coating. Poly(ethylene glycol) (see Table 2 – P23) grafted poly(acrylic acid) was adsorbed as a terminal layer onto a double layered LbL film of poly(diallyldimethyl ammonium chloride)/poly(acrylic acid) and poly(allylamine hydrochloride)/poly(acrylic acid) and deposited onto poly(dimethylsiloxane) and analysed with S. cerevisiae. Although poly(diallyldimethyl ammonium chloride)/poly(acrylic acid) and poly(allylamine hydrochloride)/poly(acrylic acid) showed minor anti-adhesive properties, the PEG grafted poly(acrylic acid)-terminated poly(diallyldimethyl ammonium chloride)/poly(acrylic acid) film was less adhesive by at least 2 orders of magnitude compared to bare poly(dimethyl siloxane).83
Antifouling coatings can be generated by zwitterionic polymers which repel microbes due to creation of an interstitial water/hydrogen-bonded network with a water layer adhering to the surface. Phosphobetaines, sulfobetaines and carboxylbetaines are the typically used zwitterionic polymers. Zhu et al.84 fabricated zwitterionic polymers including multilayers via the layer-by-layer technique on a poly(vinyl alcohol) thin-film nanofibrous composites with electrostatic interactions between poly(sulfobetaine methacrylate) (Table 2– P24) and tannic acid with coating of a filtration membrane.
Poly(acrylic acid) is one of the mostly widely used counter block polyanions in LbL self-assembled films but shows major changes in forms between pH 3 to pH 6. Tang et al.85 coated polysulfone microfiltration membranes with poly(allylamine hydrochloride) and poly(acrylic acid) (Table 2 – P25) at pH 3 to investigate their bacteriostatic properties. A single bilayer of poly(allylamine hydrochloride) and poly(acrylic acid) coated onto the membrane was used to analyse E. coli deposition kinetics to understand the bacteriostatic properties of the polyelectrolyte coted membrane. There was an approximately, 3-fold reduction in the number of E. coli found on the surface after coating, with the antiadhesive property of the modified membrane based on the highly swelling and hydrated polyelectrolyte coating preventing bacterial adhesion.
Gîfu et al.86 investigated the antimicrobial activity of a 20-mer LbL film of hydrophobically modified sodium poly(acrylate) (PAC18Na) complexes, with various chain lengths of cationic surfactants (decyl, dodecyl, tetradecyl and octadecyl trimethyl ammonium bromide) and poly(diallyldimethyl ammonium chloride) against S. aureus, E. coli, P. aeruginosa, and C. albicans. The best activity was found against S. aureus, with films of sodium poly(acrylate) complexed with tetradecyl and octadecyl trimethyl ammonium bromide, while poly(diallyldimethyl ammonium chloride) displayed total inhibition of growth after 2 h. P. aeruginosa showed greater resistance to the antimicrobial activity of the films, but after 6 h, films of sodium poly(acrylate) complexed with octadecyl trimethyl ammonium bromide and poly(diallyl dimethyl ammonium chloride) inhibited its growth. C. albicans was the least sensitive to the antimicrobial activity of the films with octadecyltrimethyl ammonium bromide complexed poly(acrylate) and poly(diallyldimethyl ammonium chloride) being the only film showing moderate inhibition of fungal growth after 24 h of exposure.
Gibbons et al.87 built a new family of layer-by-layer coatings using Nafion (a Teflon-like backbone bearing sulfate groups) (see Table 2 – P26) the enzyme lysozyme and chitosan. (Nafion/Lysozyme)6, (Nafion/Chitosan)6, and (Nafion/Lysozyme/Nafion/Chitosan)2 coatings showed excellent inhibition of E. coli and S. aureus growth (>99.9% compared to 58% for Nafion alone).
1.1.3.3. Bactericidal and bacteriostatic PEMs. There are examples of PEMs that exhibit both bactericidal and bacteria-adhesion resisting properties. Martins et al.88 prepared chitosan-based multilayer films with plant-derived polyionic polysaccharides Carrageenan (iota-carrageenan) and pectin (Table 2 – P27) and evaluated them against S. aureus and P. aeruginosa as cytocompatible antibacterial coatings. The protonation of the amino groups on chitosan being a key factor in the antimicrobial activity of the polyelectrolyte films, while the hydrophilicity of the polysaccharides in the PEMs would also reduce microbial adhesion. 15 layers of each polymer (around 16 ± 0.9 nm total thickness) were deposited onto oxidized glass substrates at pH 5.0 (X-ray photoelectron spectroscopy showed that the –NH3+ peak intensities on the surface of the pectin-chitosan film were higher than on the iota-carrageenan-chitosan film showing binding). The bactericidal action of the film was observed after 6 h for P. aeruginosa and 24 h for S. aureus. The same group89 also prepared hydrophilic, bactericidal, and anti-adhesive PEM LbL films using polycationic tannin (see Table 2 – P28) as a replacement for chitosan, and the polysaccharides, Carrageenan (iota-carrageenan) and pectin. Here the cationic NH3+ and phenolic moieties were argued as being the main reasons for their potent bactericidal action against S. aureus and P. aeruginosa, while the anionic hydrophilic components of the polysaccharides, (due to the sulfate groups of iota-carrageenan and the carboxylate group of pectin) gave rise to the bacteriostatic properties of the PEMs.
Hoyo-Gallego et al.90 built chitosan/hyaluronic acid (Table 2 – P29) bactericidal and bacteria repelling LbL films on poly(ethylene terephthalate). Without the PEM coating, the number of viable bacteria on the poly(ethylene terephthalate) surface was 1.3 × 105 CFU cm−2 while after coating with 10 layers of chitosan/hyaluronic acid, E. coli viability on the coated surface decreased significantly (8 CFU cm−2). In addition, the stability of the film was robust due to the presence of lysozyme and hyaluronidase and was stable in phosphate buffer. The authors suggested that the hydrophilic character of hyaluronic acid and the bactericidal nature of chitosan made this film a suitable candidate for biomedical coatings.
Although electrostatic interactions are favourable to design layer-by-layer deposition of polymers, Xu et al.21 produced a layer-by-layer multilayer deposition showing anti-fouling, antimicrobial, and biocorrosion inhibition from polyethyleneimine-β-cyclodextrin and ferrocene-modified chitosan via host–guest interaction chemistry on stainless steel. β-Cyclodextrin is the most popular host that able to guest various molecules due to its hydrophilic internal cavity. Similarly, ferrocene is also a widely used guest molecule for β-cyclodextrin. Thus, PEI and chitosan were functionalized with these host–guest groups. Then, poly(dopamine) anchored stainless steel surfaces were coated by alternately dipping into polyethyleneimine-β-cyclodextrin and ferrocene-modified chitosan solutions at pH 5. This took place 1, 5 and 11 times with alternative deposition of PEI and chitosan solutions. The antimicrobial and antifouling efficacies of the multilayer coatings were tested on bacteria (Pseudomonas sp. and S. aureus), microalgae (A. coffeaeformis), and barnacle cyprids. Tests revealed that as the number of host–guest assembled bilayers were increased, the antimicrobial and anti-biocorrosion performance were improved. Only a small number of viable bacteria adhered on the 11-bilayered surface with their antifouling properties much better than those compared to other assembled surfaces. The multilayer coatings were also found to be stable and durable after 35 days immersed in seawater. These environmentally friendly coatings could be useful for combatting biofouling and biocorrosion in marine and aquatic environments.
1.2. Polymers covalently immobilized onto the surfaces
Antimicrobial surfaces can also be created using covalent bonds between polymers and various surfaces is such a route to polymerize/attach functional polymers onto surfaces.91 For example Su et al.92 designed a surface by constructing a bottom bactericidal layer (800 nm) consisting of the monomer (N-(4-vinylbenzyl)-N,N-dimethylamine) and a crosslinker (ethylene glycol diacrylate) with a second monomer (vinyl pyrrolidone) introduced to give a hydrophilic layer. Poly(N-(4-vinylbenzyl)-N,N-dimethylamine) coatings exhibited more than 99.9% killing of both E. coli and B. subtilis regardless of the incorporation of vinyl pyrrolidone on the surface. However, incorporation of vinyl pyrrolidone led to much better antifouling resistance and improved biocompatibility.Fluorinated polycationic polymers were attached onto textiles by Song et al.93via initiated chemical vapor deposition, forming poly(dimethylaminomethylstyrene-co-1H,1H,2H,2H-perfluorodecyl acrylate). The cationic fluorinated polymer coated surface reduced by some 3-log units the attachment of methicillin resistant S. aureus and E. coli (99.9% killing efficiency). The antimicrobial mechanism of the polymer was explained by their similarity with other cationic polymers. In addition, the hydrophobic group of the polymer was attributed to the greater binding efficacy shown to E. coli resulting in better penetration of the polycations. The polymer surface was tested on lentivirus (an enveloped virus) and again, due to electrostatic interactions between positively charged groups on the polymer surface and the “negatively charged lentivirus” as well as hydrophobic interactions with the viral “membrane”, the cationic fluorinated polymer surface damaged the viral structure.
Among the various covalently bonded surfaces, polymer brushes are of particular interest as non-leachable coatings in biomedical applications due to their mechanical stability and tuneable thicknesses. Such grafted polymer chains can be generated using a variety of polymerisation techniques and also allow subsequent modification with other functional groups.94
1.2.2.1 Bactericidal polymeric brush surfaces. Metallic implants and devices used in clinical applications tend to generate bacterial associated infections. To combat bacteria associated infection on titanium alloys, Peng et al.97 designed a surface coating made of a novel phosphonate/active ester block polymer composed of phosphonate units (degree of polymerization of 29) that served as the metal anchoring segment and NHS active ester units (various degree of polymerization: 7, 29, and 64) which were conjugated to poly(hexamethylene biguanide). Although all copolymers of poly(diethyl(methacryloyloxymethyl)phosphonate-b-N-hydroxysuccinimide methacrylate) showed great antimicrobial activity (following conjugation) the polymer having 64 active ester repeat units, and then functionalized with poly(hexamethylene biguanide) showed the best antimicrobial activity, killing and inhibiting 100% of E. coli and S. aureus. The ruptured membrane along with intracellular matrix release indicated that the polymer showed bactericidal action due to interaction of the guanidine groups with the various negatively charged components in/of the bacteria with no suppression of mammalian cell viability. In vivo data showed that the polymer coating significantly inhibited colonisation of bacteria on implants and consequently decreased levels of bacterial associated infection.
Poly(ethylene terephthalate)98 is perhaps one of the most widely used synthetic polymers due to its low cost, and mechanical and chemical stability. It is also inert and hence often biocompatible. Thus, PET has seen utilization in heart valves, vascular grafts, surgical meshes, and artificial ligaments. However, PET can be contaminated by microbes as it does not have any inherent antimicrobial properties. Therefore, Cao et al.99 covalently grafted poly(hexamethylene guanidine hydrochloride) – a well-known cationic polymeric bactericide that shows potent killing activity against a range of pathogens with a mechanism of action mediated via electrostatic interactions with negatively charged cell surface components. Here, a polymeric brush surface was developed using poly(ethylene terephthalate) that was modified via aminolysis with ethylenediamine. This amine was then reacted with ethylene glycol diglycidyl ether that had itself been reacted with poly(hexamethylene guanidine hydrochloride). Uncoated and amine group terminated PET surfaces showed no bactericidal activity whereas the polymer brush coated PET surfaces showed excellent bactericidal activity against E. coli and S. aureus.
Larikov et al.100 prepared glass surfaces with covalently immobilized poly(allylamine) (via 3-glycidoxypropyltrimethoxy silane modification of the glass followed by reaction with free amino groups), which showed bactericidal activity against S. epidermidis and S. aureus (eliminating ∼97%) and P. aeruginosa (88%). Comparison with electrostatically bound poly(allylamine) surface vs. covalently attached polyallylamine surface showed that the killing efficiency of the covalently attached surfaces were much higher with improved robustness and that surface charge was more important than the chain lengths of the polymer.
It is known that cationic polymers bearing quaternary ammonium salts along the polymer chain display high killing of pathogens. Li et al.101 prepared a stable antimicrobial coating with two natural polymer derivatives, agarose and quaternized chitosan via the “grafting-to” method onto polymer films [polyurethane and poly(vinyl fluoride)], and titanium foil. Polymers were covalently grafted via a “thiol-ol” reaction (conjugation under UV irradiation in the presence of oxygen with dimercaprol serving as both an anchor and as a crosslinker between the surface and grafted polymer). Due to the antiadhesive nature of agarose and the bactericidal nature of chitosan, the prepared surfaces thus offer two different modes of action. The agarose grafted surfaces inhibited biofilm formation of both P. aeruginosa and S. aureus (99% compared to the uncoated surfaces). In addition, agarose and chitosan grafted surfaces maintained their antibacterial activities for 30 days after repeated ethanol treatment or autoclaving. When the quaternized chitosan grafted polyurethane or poly(vinyl fluoride) surfaces were “contaminated” with drops of viable S. aureus and P. aeruginosa, a negligible number of bacteria remained alive after contact (in contrast the surfaces were non-cytotoxic to 3T3 fibroblasts). Again, the quaternized ammonium groups (in this case on the chitosan) mediated contact killing of bacteria.
Correia et al.102 developed chitosan scaffolded surfaces functionalized, with N,N-dimethyldodecylamine quaternized poly(2-oxazoline)s using 2-methyl-2-oxazoline, 2-ethyl-2-oxazoline or 2-bisoxazoline with the ‘grafting-from’ method performed, using super critical CO2 as a solvent. They found that quaternized poly(2-methyl-2-oxazoline) grafted chitosan scaffold was the most effective against S. aureus and E. coli (killing almost 100% in 3 min), which also prevented of biofilm formation. Moreover, this scaffold was able to purify water samples taken from different sources and performed well over multiple cycles.
Koufakis et al.103 investigated poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) brushes quaternized with various alkyl chain lengths as biocidal coatings with surface-initiated atom transfer polymerization used to grow polymer chains on silicon wafers. Three different molecular weight of poly(2-(dimethylamino)ethyl methacrylate) (Mw: 30 kDa, 81 kDa and 110 kDa) were synthesized and the polymers were quaternerised with various alkyl halides and tested against E. coli and B. cereus. (see Fig. 9). Polymers having short alkyl chains (e.g., methyl, ethyl, and propyl) showed a 100–1000-fold decrease in bacterial adhesion, but with 99% killing of adhered bacteria, while non-quaternized and long alkyl chains (more than 6 carbons) showed little reduction in bacteria colonisation. They explained this phenomenon as conformational alteration in the polymer due to formation of a “cumbersome hydrophobic barrier” with the long alkyl chains preventing the interaction of quaternized cationic moieties with the bacteria. On the other hand, polymers with short alkyl chains were able to move more freely, and the positive charges could interact with the negatively charged bacterial cell membrane leading to perforation and lysis. Field emission scanning electron microscopy images (Fig. 10) showed the bactericidal effect of the low molecular weight, methyl quaternized, PDMAEMA brushes with a collapsed bacterial morphology with damaged membranes, while non-quaternized or octadecane quaternized polymers showed intact bacteria adhesion.
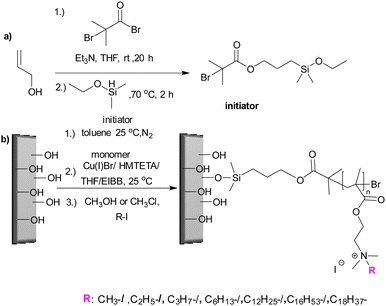 | ||
| Fig. 9 (a) Silyl-based initiator synthesis; (b) initiator immobilisation and synthesis of PDMAEMA brushes and quaternization with various alkyl chain lengths via surface initiated-ATRP.103 Reproduced with permission from {Koufakis, E.; Manouras, T.; Anastasiadis, S. H.; Vamvakaki, M. Film Properties and Antimicrobial Efficacy of Quaternized PDMAEMA Brushes: Short vs Long Alkyl Chain Length. Langmuir 2020, 36, 3482–3493}. Copyright {2020} American Chemical Society. | ||
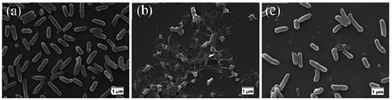 | ||
| Fig. 10 Field emission scanning electron microscopy images of E. coli adhesion onto the surfaces of low molecular weight PDMAEMA brushes: (a) non-quaternized PDMAEMA; (b) methyl quaternized PDMAEMA and (c) octadecyl quaternized PDMAEMA.103 Reprinted with permission from {Koufakis, E.; Manouras, T.; Anastasiadis, S. H.; Vamvakaki, M. Film Properties and Antimicrobial Efficacy of Quaternized PDMAEMA Brushes: Short vs Long Alkyl Chain Length. Langmuir 2020, 36, 3482–3493}. Copyright {2020} American Chemical Society. | ||
Using similar polymerization techniques Yandi et al.104 prepared poly(2-(dimethylamino)ethyl methacrylate) brushes for use against the marine alga Ulva linza and U. lactuca in order to understand the anti-algal effects of the brushes, spore adhesion and growth. In this case a self-assembled mono layer surface was formed using α-bromoisobutyrate-11-mercapto-undecane as the initiator for ATRP polymerization. Poly(2-(dimethylamino)ethyl methacrylate) brushes were anti-algal, killing zoospores upon contact as well as inhibiting the growth of spores. This phenomenon was again explained by electrostatic interactions of the negatively charged spores and the cationic groups of the polymer brushes, disrupting the membrane, a similar mechanism to that of poly(2-(dimethylamino)ethyl methacrylate) and its antimicrobial mode of action.
Lu et al.105 prepared poly(diallyldimethyl ammonium chloride) grafted onto cellulose filters whose hydroxyl groups had been reacted with 2-bromisobutyryl bromide (by esterification), thus serving as a macroinitiator in an ATRP reaction to allow the grafting of diallyldimethyl ammonium chloride. Uncoated membranes and different levels of poly(diallyldimethyl ammonium chloride) grafted onto the cellulose membrane were tested for bacterial killing by immersing into bacterial suspensions with a grafting ratio of 13 wt%, reducing bacterial numbers by 98% for S. aureus and 92% for E. coli.
Undoubtedly, contact lenses are one of the most widely used devices to correct refractive errors and maintain ocular health. However, wearing contact lens use can cause adhesion and colonization of bacteria which can develop conditions such as acute red eye, acute dry eye, microbial keratitis or corneal complications (neovascularization, edema, staining) and so on.106
Chan-Park et al.107 developed two silicone contact lens coatings via ozone-activation and thermal-polymerization. In the first coating, PEI was grafted with poly(ethylene glycol) methacrylate with the polymer tethered onto the surface via thermal polymerization (with the help of peroxide groups after ozone activation of the surface). This surface displayed good in vitro antibacterial properties against methicillin resistant S. aureus with an average log reduction of 5 (<99.99% killing) and with high mammalian cell viability. Another coating was synthesized starting from the monomers glycidyl methacrylate (for PEI grafting) and N-(3-sulfopropyl)-N-(methacryloxyethyl)-N,N-dimethylammonium betaine (as a hydrophilic unit for the coating) with polymerization based on free radical polymerisation followed by macromolecular grafting. The best polymer formulation for antimicrobial activity used 1 wt%, glycidyl methacrylate, 1 wt% of the hydrophilic betaine and 0.5 wt% PEI (Mw: 2 kDa) with a more than 6 log reduction of methicillin resistant S. aureus yet with 90% fibroblast cell viability.
Covalent immobilization of antibacterial peptides onto surfaces has gained a great deal of interest due to their fast, effective, and broad-spectrum killing action. Gao et al.108 investigated the antibacterial properties of polymer brush systems coated onto titanium surfaces with primary amines containing copolymers (e.g. poly(N-(3-aminopropyl))methacrylamide-co-(N,N-dimethylacrylamide)) synthesized by surface initiated atom transfer radical polymerization. Here, the cysteine functionalized cationic, antimicrobial peptide Tet213 (KRWWKWWRRC), was conjugated onto the copolymer brushes using a maleimide-thiol addition reaction after initial modification of the grafted chains using an N-hydroxysuccinimide ester of 3-maleimidopropionic acid. They found that the graft density of the peptide on the surface had a strong effect on the antibacterial activity of P. aeruginosa. The same group109 also tested an antimicrobial peptide tethered brush coating generated from a copolymer of N,N-dimethylacrylamide-co-N-(3-aminopropyl)-methacrylamide (5/1 monomer ratio) and the peptide Tet-20 (KRWRIRVRVIRKC) against P. aeruginosa and S. aureus. Peptide coated titanium wires showed a 5-log decrease in CFU (compared to uncoated), in addition, peptide coated wires, which were implanted into subcutaneous pockets of rats, showed a decrease in S. aureus levels by at least 85%. It is likely that the use of the D-amino acid peptide enantiomer would promote the effect, as the L-enantiomer would be degraded by proteases. The antibacterial effect here is mediated by the mixed hydrophobic/cationic peptide and the non-bonding nature of the dimethylacrylamide.
Urinary Tract Infections are the most common type of infection. In hospital settings a major cause is ureteral stents that remain in position for long periods. Unfortunately, Proteus mirabilis (P. mirabilis), which is the main urease positive pathogen in the ureteral system, starts to form biofilms which induces encrustation (urinary tract stones), hindering the flow of the urine. Later, mucosal injury occurs, and the medical device must be replaced with a surgical operation. Gultekinoglu et al.110 developed an antimicrobial PU stent upon which the cationic polymer PEI was grafted, with the stent showing a significant decrease in bacteria adhesion under static conditions. The same group111 studied two different molecular weight PEI's (1.8 and 60 kDa) grafted PU stents and also alkylated these with hexyl bromide to enhance their antimicrobial activity within a dynamic biofilm reactor, to observe bacteria adhesion, biofilm formation and encrustation. The grafted PEI affected bacteria adhesion owing to the dynamic motion of the brushes in the liquid environment with the alkylated PEI brushes disrupting the bacteria cell wall upon contact. The high molecular weight PEI grafted stent decreased biofilm formation by up to 2 orders compared to unmodified PU's, in in vitro experiments. Free Ca2+ and Mg2+ ions play a key role in the encrustation process; hence, PEI brush stents were tested in terms of deposition onto the stent surfaces (encrustation). The grafted PEI inhibited Ca2+ and Mg2+ ions deposition by 81% and 93%, respectively in in vivo animal models and showed reduced inflammation in host tissue.
1.2.2.2. Dual-functional polymeric brush surfaces. Hydrophilic polymers and zwitterionic polymers generate a hydration layer that forms a physical-type barrier that gives rise to the anti-adhesive properties of surface. This combination of a robust interfacial hydration layer and steric repulsion generates resistance to protein/bacterial binding.112–115 Such polymeric brushes can be prepared on various surface such silicone catheters and in this content, Yu et al.116 prepared an antimicrobial polydimethylsiloxane surface coating showing both antifouling and bactericidal activity against E. coli via sub-surface-initiated atom transfer radical polymerization. Firstly, a vinyl-terminated initiator was blended with polydimethylsiloxane, then 2-(dimethylamino)ethyl methacrylate was polymerized onto the entrapped initiator via atom transfer radical polymerization and then quaternized with 1,3-propanesultone and 1-bromo-undecane to obtain zwitterionic and bactericidal groups on the same polymer brush. Antimicrobial and antifouling activities of the polymer were investigated, and it was found that grafting density, grafting time and degree of quaternization and sulfobetainization of polymer had a great impact on their properties. In terms of antifouling approaches, hydrophilic polymers have been employed to form a hydration layer to prevent attachment of bacteria to surfaces, while exhibiting both microcidial and anti-adhesive actions, again with a focus on reducing catheter related infections. Zhang et al.117 developed a polymeric catheter surface with a foundation zwitterionic polymer layer (poly(3-[dimethyl-[2-(2-methylprop-2-enoyloxy)ethyl]azaniumyl]propane-1-sulfonate)) with an upper layer of poly(methacrylic acid), displaying antifouling, bactericidal and hemocompatible properties. Polyurethane surface functionalization with an initiator allowed a zwitterionic polymer to be grafted from the surface, with poly(methyl methacrylate) grafted onto the zwitterionic brush, and an antibacterial peptide coupled via amidation. The hydration layer created by the hydrophilic and negatively charged polymer brushes generated an antifouling surface active against both E. coli and S. aureus under static and hydrodynamic conditions.
It is a challenge to design a surface that can prevent bacterial infections and show antiadhesive properties while promoting tissue biocompatibility. Hoyos-Nogués et al.118 prepared a titanium surface showing both inhibition of bacterial colonization while promoting osteoblast cell attachment for dentistry applications. Thus, PEG was added by an electrodeposition process, followed by the immobilization of peptides that promoted both cell proliferation (Arg-Gly-Asp (RGD)) and bactericidal action (adding the peptide LF1-consisting of 11 residues from the amino-terminus of lactoferrin). The antibacterial activity was tested against S. sanguinis – a primary colonizer in oral biofilms. Due to the low bio-fouling property of the PEG coating on the surface, bacterial attachment was significantly decreased on the PEG and PEG-peptide coated surfaces. In addition, SEM images showed PEGylated surfaces inhibited the formation of filamentous appendages (called fimbria) that lead to bacteria adhesion to solid surfaces and later biofilm formation. Furthermore, the PEG-peptide coated surfaces increased the percentage of dead bacteria, while reducing the adhesion of bacteria compared to a simple PEG coated titanium surface.
Fu et al.119 prepared a novel antimicrobial mix brush surface based on poly(N-hydroxyethyl acrylamide) and poly(trimethylamino)ethyl methacrylate chloride on silica surfaces. The mixed polymer brush coating was generated through surface-initiated atom transfer radical polymerization (for the antifouling monomer) and surface-initiated/mediated polymerization (for the cationic monomer). The 100% poly(N-hydroxyethyl acrylamide) surface showed excellent antifouling properties, while the 100% cationic poly(trimethylamino) ethyl methacrylate chloride surface showed bactericidal activity with dead bacteria on the surface. In order to prevent attachment of dead bacteria while maintaining bactericidal activity, an in a ratio of initiators 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used to give a mixed polymer brush having both antifouling and antibacterial activity against Gram-positive and Gram-negative bacteria. The mixed brush surface reduced the attachment of dead bacteria by ∼10 fold, while maintaining a high kill level (the killing ability of the cationic brushes based on the interaction of the polymeric cationic groups with the various anionic components of the bacterial membrane/wall causing distortion and leakage of intracellular components, resulting in death of the bacteria).
1 was used to give a mixed polymer brush having both antifouling and antibacterial activity against Gram-positive and Gram-negative bacteria. The mixed brush surface reduced the attachment of dead bacteria by ∼10 fold, while maintaining a high kill level (the killing ability of the cationic brushes based on the interaction of the polymeric cationic groups with the various anionic components of the bacterial membrane/wall causing distortion and leakage of intracellular components, resulting in death of the bacteria).
An antimicrobial surface was created by He et al.120 with the mixing of two types of dopamine-modified polymers (inspired by mussel adhesion chemistry). Thus, butyl, octyl and dodecyl quaternized poly(2-(dimethylamino)ethyl methacrylate) and poly(sulfobetaine methacrylate) was synthesized via ATRP with dopamine-modified with a 2-bromoisobuty group as an initiator. The effect of the N-alky chain length, of the quaternized ammonium group of poly(2-(dimethylamino)ethyl methacrylate) on antimicrobial activity of a mixed brush surface was noted to decrease with increasing chain length, arguably because of the shielding effect of the N-alkyl chains on the hydrophilicity of the polymer. Of note, [poly(sulfobetaine methacrylate)/poly(2-dimethylbutylammonium)ethyl methacrylate] mixed brushes exhibited bactericidal and antifouling properties. When poly(2-dimethylbutylammonium)ethyl methacrylate content was in the range of 10–50% on the surface, the killing efficiency of polymer brushes against S. aureus was reached to 74–92%. Longer N-alkyl chains polymers (e.g. octyl quaternized) displayed a killing level of 69–94% when the quaternized content was in the range of 10–30%. For, poly(sulfobetaine methacrylate)/dodecyl quaternized poly(2-(dimethylamino)ethyl methacrylate) up to 10% was needed for good bactericidal and antifouling activity against S. aureus.
1.2.2.3. Self-adapting polymeric brush surfaces. Self-adaptable surfaces that can changes their antimicrobial properties from bactericidal to antifouling by a stimuli-responsive strategy have been an attractive development that has allowed the preparation of new antimicrobial surfaces coatings. Intravenous catheters are the one of the most widely used devices in clinics, unfortunately, catheter-associated infectious are associated with high levels of morbidity and mortality are very common. Thus, innovative catheter coatings are essential. Zhang et al.121 prepared self-adaptive antibacterial surface with switching on of antibacterial action in the presence of bacteria. The first block of the polymer brush coating was a bactericidal poly(2-(dimethyldecylammonium)ethyl methacrylate) using monomer 2-(dimethyldecylammonium) ethyl methacrylate grafted via a surface-initiated atom transfer polymerization onto a polyurethane (to had been coupled to initiator). The second block was hydroxyethyl methacrylate, with the hydroxyl group of the polymer oxidized (to aldehydes) to allow conjugation of amine terminated polyethylene glycols (PEG's) via Schiff base formation. In the presence of bacteria, the imine bonds hydrolyse due to bacterial metabolism and the creation of a locally acidic microenvironment, thus releasing the upper PEG layer, and thereby revealing the lower bactericidal layer that eliminates the bacteria and inhibits infection. The polymer brush coated surfaces reduced by 97% the level of S. aureus binding in the short term (10 h) and killed 82% of bacteria over 24 hours. In addition, the antibacterial activity of this self-adaptive surface was tested under hydrodynamic conditions and showed high antifouling efficiency (88% reduction of bacteria adhesion). By applying a similar strategy, an arginine-based switchable surface coating was generated, turning from bactericidal to antifouling.122 The key here being the use of L-arginine methyl ester-methacryloylamide polymer brushes grafted by surface-initiated reversible addition−fragmentation chain-transfer. Here, the glass surfaces were modified with polydopamine via catechol coordination, and then functionalized with a chain transfer agent to allow RAFT polymerization. Subsequent polymerization was carried out to allow generation of a polymer brush surface. Due to the guanidinium groups on the polymer brushes, the surfaces showed bactericidal activity against S. aureus and E. coli. Upon hydrolysis of the ester the surface changed from a bactericidal state (due to cationic groups) to an antifouling state due to the zwitterionic groups thus preventing the adhesion of S. aureus and E. coli and A. coffeaeformis (Fig. 11).
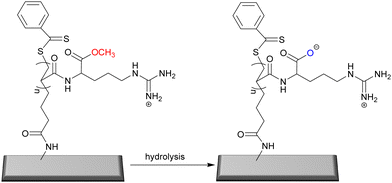 | ||
| Fig. 11 The hydrolysis of L-arginine methyl ester-methacryloylamide based-brushes used to generate a “switch-on” antifouling surface by ester hydrolysis.122 Reprinted (adapted) with permission from {G. Xu, X. Liu, P. Liu, D. Pranantyo, K. Neoh and E. Kang, Langmuir, 2017, 33, 6926–6936} Copyright {2017} American Chemical Society. | ||
pH-responsive tannic acid-scaffold binary polymer brushes have been prepared as coatings. Here azido-modified tannic acid was prepared and coupled as a co-polymer that had been generated using a dibenzocyclooctyne modified chain transfer agent via RAFT polymerization as well as cationic dibenzocyclooctyne modified poly-lysine via a copper-free azide−alkyne cycloaddition reaction (these were coated onto stainless steel surfaces via coordination chelation chemistry). The antimicrobial activity of the surface was tested with S. epidermidis and E. coli and A. coffeaeformis. Since poly(2-diisopropylaminoethyl methacrylate) shows reversible pH switching behaviour, such that when the pH decrease (below the pKa value of 6.3) the poly(2-diisopropylaminoethyl methacrylate) chains swelland the zwitterionic polymer segment becames dominant, showing antifouling properties. However, when the pH increases, the poly(2-diisopropylaminoethyl methacrylate) chains collapse and the poly-lysine chains became “dominant/exposed” showing bactericidal action.123
Wu et al.124 developed a polymeric mixed brush surface that altered bacterial attachment in response to pH. Thus, vinyl and alkyl bromine functionalized gold surfaces were prepared and poly(2-(dimethylamino)ethyl methacrylate) was generated via thermally initiated free radical polymerization and then visible light photoinitiated polymerization for poly(acrylamide phenylboronic acid) grafting. At low pH's poly(2-(dimethylamino)ethyl methacrylate) have protonated tertiary amino groups and the surface bound S. aureus, whereas at higher pH values (pH 9.0) the ammonium groups of the polymer become deprotonated and the boronic acids become boronates making the surface negatively charged and bacteria repellent.
Although cationic groups of polymers typically show high antimicrobial efficacies, they may show toxicity to mammalian cells and promote biofilm formation, due to the non-removal of dead microbes from the surface. For this reason, switchable polymer brush surfaces have the vision of creating an ideal “cleaning” antimicrobial surface. Yan et al.125 designed a hierarchical polymer brush surface having a “zwitterionic” outer most layer with an underlying polycationic bactericidal polymer. Thus [3-(methacryloylamino)propyl]dimethyl(3-sulfopropyl)ammonium hydroxide inner salt and 2-(dimethylamino)ethyl methacrylate were graft polymerized onto silicon wafers bearing photoiniferter moieties. The cationic elements of the polymer brush were further modified with 1-bromoethane to give a stable cationic charge (as quaternary ammonium salt). Since medical devices can be contaminated by air-borne bacteria antimicrobial surface should also be effective against microbes in the dry state. Here, the synthesized polymer killed ∼76% and ∼95% of S. aureus and E. coli. Here, the mechanism of the polymers is based on the quaternary ammonium groups interacting with the bacteria membrane/cell wall leading to lysis of bacteria with the sulfonated brushes in a collapsed position in the dry state. Under wet conditions such as body fluids containing planktonic bacteria, the sulfonated layer bearing polymer brushes prevent the accumulation of bacteria (reduction of ∼98% and ∼97% for S. aureus and E. coli) and showed high killing rates (∼80% and ∼77% for S. aureus and E. coli) relative to surfaces prepared with polycationic polymer brushes alone. In addition, the polymer showed that bacteria attachment onto the surface was prevented over longer term. Due to the sulfonated groups of the brushes, the cationic groups stayed physically hindered from mammalian cells, thus the toxicity induced by cationic groups of the polymers was prevented.
Chen et al.126 created a hierarchical polymeric brush surface made up of cationic units [poly(1,3-bis-N,N-dimethyl-N-octyl-ammonium)-2-propylacrylate dibromide] and a zwitterionic poly(sulfobetaine methacrylate) antifouling layer via surface-initiated atom transfer radical polymerization. In the design the graft density of the cationic polymer was changed to allow evaluation of the effect on its self-cleaning ability. The optimum graft density of the brush was approximately 61 nm and prevented binding of over 70% of E. coli of S. aureus compared to the bare surface. The mode of action is believed to be due to high charge density and long alky chains of cationic polymer unit, the brushes could bind bacteria, leading to the breakage of the bacterial cell membrane and lysis of the bacteria.
2. Polymer solutions as antimicrobial agents
Antibiotics provide powerful ammunition for mankind to tackle pathogenic diseases. However, widespread misuse and poor stewardship has led to widespread resistance, specifically, multidrug resistant Gram-negative bacteria whose outer membrane acts as a barrier to many antibiotics. Therefore, new solutions are essential, and polymers have emerged as part of the solution to combat such diseases, with key learnings coming from natural host defence peptides and polymers that typically interact with both the negatively charged (phospholipid) and hydrophobic elements of the bacterial outer membrane (Gram-negative). Thus, if synthetic polymers can be designed and fabricated such that they have facial amphiphilicity effective interactions with the bacterial membranes will occur. Rahman et al.127 synthetized polymers having intrinsic facial amphiphilicity using bile acids (cholesterol-derived amphiphilic steroid acids). Initially monomers were synthesized by esterification with three different bile acids (lithocholic, deoxycholic, and cholic acid) and hydroxyethyl methacrylate and homopolymers synthesized via RAFT polymerization. These homopolymers were further modified by esterification (lithocholic, deoxycholic, and cholic acid having one, two and three available alcohol groups) with different lengths of bromoalkanoyl chloride and the alkyl bromide groups on the subsequent polymers substituted with trimethylamine to form quaternary ammonium polymer side chains. Homopolymers of cholic acid showed the best activity against S. aureus, E. coli and P. aeruginosa while the cholic acid derived polymers with a pentyl spacer between the ester and the quaternary ammonium group exhibited the best antimicrobial properties (MIC values 7–15 μg mL−1). The cholic acid polymers bearing five methylene spacers and having a molecular weight around 10 kDa displayed the most effective antibacterial action compared to higher molecular weight polymers. The cationic groups on each cholic acid unit interacted with the outer membrane of the bacteria via electrostatic interactions and the hydrophobic cholic acid interlacing into the membrane lead to bacterial death. Recently, the same group128 used nanostructured polymers with facial amphiphilicity to combat multidrug resistant bacteria. Thus, copolymers of cholic acid methacrylate (2-methacryloyloxy)ethyl cholate, and poly(ethylene glycol)methacrylate were synthesized via reversible addition–fragmentation chain transfer polymerization. Copolymers bearing 90 and 70 mol% of cholic acid formed spherical particles, while copolymers at 58 and 47 mol% formed rod like structures in water with an average diameter of 150–400 nm. The spherical particles inhibited the growth of Gram-negative bacteria (minimum inhibition concentration for the polymer bearing 90% cholic acid was 13 and 10 μg mL−1 against E. coli and P. aeruginosa respectively), while the rod-shaped polymers were effective against S. aureus, with the killing mechanism associated with membrane disruption as observed via SEM.Zhou et al.129 designed host-defence peptide mimics via glycine-pendent polyoxazolines with four polymers varying in lengths (from 6 to 40-mers) synthesized. The best polymers were found to contain 20 monomers with an MIC value between 3–12 μg mL−1 (against S. aureus) (including drug resistant strains and S. epidermidis and B. subtilis). Importantly, S. aureus did not show resistance to this polymer and the antibacterial mechanism was explained as the polymer entering the cytoplasm without harming the bacterial membrane and then interacting with bacterial DNA and subsequent triggering of the generation of reactive oxygen species (ROS). Although host defence polycationic antimicrobial peptides are attractive due to their low resistance potential, there are limitations for clinical use due to cost, limited activity, and poor stability. Therefore, synthetic polymers are an attractive alternative. Specifically, cationic polymers have been well explored over the years, playing with composition, architecture, and synthesis. Venkatesh et al.130 investigated the antibacterial actions of commercially available cationic polymers ε-poly-lysine, α-poly-L-lysine, α-poly-L-ornithine, α-poly-D-lysine, α-poly-L-arginine, poly(allylamine), linear polyethyleneimines and branched polyethyleneimines (Fig. 12). All polymers showed antibacterial activity against P. aeruginosa, S. aureus, and C. albicans, yet were not effective as cationic antiseptics. Among all of these, ε-poly-lysine and linear polyethyleneimine showed the best activity, additionally, ε-poly-lysine showed strong antimicrobial activity against ocular fungal pathogens such as Fusarium comparable to the ophthalmic antifungal natamycin.
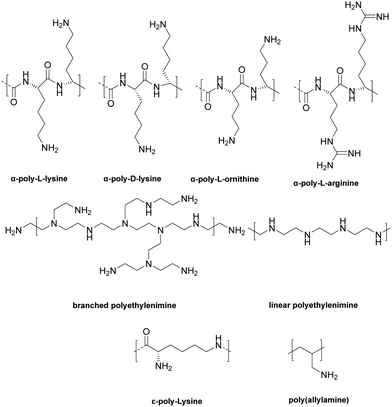 | ||
| Fig. 12 The structures of a broad family of commercial, cationic, antimicrobial polymers, that are used in applications ranging from food preservation to the pharmaceutical industries.130 Reproduced from ref. 130 with permission from PMC. | ||
Another natural antimicrobial agent is the cationic peptide, ε-poly-L-lysine (ε-PL). However, its antimicrobial mode of action is uncertain, and, in the literature, there are various inhibition concentration values although the difference is often based on the source of the peptide. Tan et al.131 has helped to clarify the antibacterial action and mechanism of ε-poly-L-lysine towards S. aureus. In their study, bacteria were treated with 250, 500 and 750 μg mL−1 of polymer, with increasing concentration resulting in greater collapse of the bacteria structure. Using Raman spectroscopy, they showed that ε-poly-L-lysine passed through the membrane and interacted with teichoic acid found in the peptidoglycan layer of the cell wall. The peptidoglycan structure was subsequently destroyed by the polymer, with the polymer also interacting with the plasma membrane leading to an increase in plasma membrane permeability.
Chitosan is extensively used in antimicrobial applications; however, its solubility can be problematic at physiological pH's. The amine group at C-2 and the hydroxyl group at C-6 enable functionalization and Su et al.132 prepared chitosan derivatives via modification with arginine at the C-6 position. Due to the amine groups of chitosan and the guanidyl group of the added arginine group the chitosan derivative showed improved solubility as well as antibacterial activity with minimum inhibition concentrations of the polymer being 780 μg mL−1 for S. aureus and 312 μg mL−1 for E. coli.
Cyclodextrins are cyclic oligosaccharides of varying sizes (common ones named α, β and γ). β-Cyclodextrin is commonly used due to its hydrophobic interior and hydrophilic exterior, and its availability. β-Cyclodextrin was grafted onto chitosan by Ding et al.133 and tested on S. xylosus and E. coli. SEM images revealed severely damaged, atrophied, sunken and disrupted cell membranes and cell lysis. The bactericidal mechanism of polymers is based on the quaternized ammonium groups of chitosan under acidic conditions that interact with the negatively charged bacteria membrane/cell wall components. Lower levels of β-cyclodextrin modification showed better bactericidal activity and the β-cyclodextrin modification promoted drug (doxorubicin) uptake by S. xylosus, while improving the aqueous solubilities of sulfadiazine, sulfamonomethoxine and sulfamethoxazole.
Phillips et al.134 investigated cationic polymers based on poly(2-(dimethylamino)ethyl methacrylate) (4.5 kDa, 6.1 kDa and 11.2 kDa), poly(2-(dimethylamino)ethyl acrylate) (11 kDa and 3.2 kDa) and poly(2-aminoethylmethacrylate) (11.2 kDa) synthesized via reversible addition−fragmentation chain transfer polymerization against M. tuberculosis (Fig. 13). Among all the polymers, poly(2-(dimethylamino)ethyl methacrylate) showed the most potent activity (minimum inhibition concentrations as low as 30 μg mL−1 against M. smegmatis), while the lowest molecular weight poly(2-(dimethylamino)ethyl methacrylate) exhibited antibacterial activity against E. coli and P. putida. Poly(2-(dimethylamino)ethyl methacrylate) bound to mycobacteria but did not change the integrity of the cell wall.
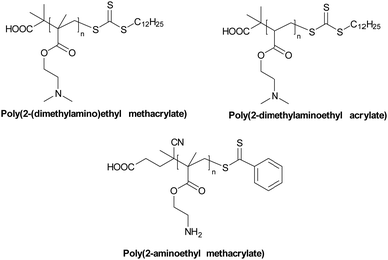 | ||
| Fig. 13 Structures of the cationic polymers generated via RAFT polymerization and screened against M. smegmatis. Note: all the polymers will be protonated at physiological pH.134 Reproduced from ref. 134 with permission from PMC. | ||
Thoma et al.135 investigated the antimicrobial activity of poly(aminoethyl methacrylate)s. Polymers were synthesized via RAFT polymerization of the monomer N-(tert-butyloxycarbonyl)aminoethyl methacrylate and the RAFT agent 2-cyanoprop-2-yl ethyl dithiocarbonate. Three different molecular weight polymers (2.1, 2.7, 3.2 kDa) were deprotected with TFA to give the primary ammonium group-based polymers and were evaluated on Gram-negative and Gram-positive bacteria including methicillin-resistant S. aureus. Polymers showed more of an effect on the inhibition of the growth of Gram-positive bacteria (MIC values lower than 100 μg mL−1) when compared to the Gram-negative bacteria (MIC values around 700 μg mL−1). Importantly the polymers were active in the presence of fetal bovine serum (serum binding can cause a loss of antimicrobial activity) and the polymers showed higher killing rates compared to the antibiotics, norfloxacin and mupirocin. The highest molecular weight polymers caused a 3-log reduction in the number of viable bacteria after 60 min and showed limited cytotoxicity and hemolysis at high concentrations of polymer. In vivo using a cotton rat nasal S. aureus colonization model, the low molecular weight polymer (used at 2× its MIC) effectively reduced the number of viable bacteria to the same level as mupirocin (when used at 2000× its MIC) (an antibiotic used to eradicate S. aureus nasal decolonization).
Fungi have a significant impact/effect on surface colonization e.g., medical devices. Upon starting colonization of a surface, fungi start to build complex, robustly attached films that can often be different colours due to melanisation of the biofilms. Tellez et al.136 synthesized, by RAFT polymerization, a poly(2-(dimethylamino)ethyl methacrylate) library that was screened against various fungi using three different molecular weights of polymer (9.7, 17.8 and 47.4 kDa) with and without quaternization (using various ratios of iodomethane and 1-iodobutane). Among the fungi explored, only one, C. lunata did not display complete inhibition when exposed to any of the synthesized polymers. Although all polymers possessed activity against multiple fungi, even when non-quaternized, the lowest molecular weightquaternized polymers showed the best fungicidal activity, presumably the lower molecular weights were able to better diffuse through the fungal cell wall before disruption of the cell membrane.
Mukherjee et al.138 synthetized branched polyethyleneimine derivatives containing alkyl, alkyl ester and alkyl amide groups and evaluated them against drug resistant Gram-positive bacteria and fungi. Branched PEI was initially N-methylated and then further alkylated to form amphiphilic cationic polymers (to synthesize the ester and amide alkylating agents; alcohols and amines were reacted with bromoacetyl bromide, and the products subsequently used to alkylate the N-methylated PEI). The modified PEIs with short aliphatic ester or alkyl chains did not show antimicrobial activity, whereas the hexyl derivatives showed activity against S. aureus, E. coli and C. albians. Specifically, the hexyl amide functionalized PEI showed the best activity displaying MIC values of 2–4 μg mL−1 against methicillin-resistant S. aureus, 8 μg mL−1 against E. coli and 4 μg mL−1 against C. albicans. Since the hexyl amide functionalized polymers displayed optimal activity, they were further explored. Killing of S. aureus, (>5 Log reduction) was found within 4 h at 2× MIC. The hexyl amide functionalized polymers were able to eradicate 80% of biofilms of clinically isolated S. aureus at 32 mg mL−1. The polymer also exhibited potent activity against both metabolically inactive stationary phase bacteria and antibiotic-resistant bacteria due to non-specific interactions with the cationic/lipophilic polymer with the bacterial killing. Of note, this polymer maintained its activity upon incubation in complex physiological fluids and did not show any propensity to develop resistance over 15 days (Fig. 14–17).
The pH of normal skin is around 5.4–5.9 (which usually prevents the growth of bacteria), while it is known that the pH of infected skin is close to neutral. Hong et al.139 therefore synthesized a pH responsive cationic, amphiphilic random copolymer as a topical antibacterial agent and tested it against drug resistant S. aureus. The methacrylate copolymer consisting of aminobutyl methacrylate and ethyl methacrylate (Mw: 2600 Da), synthesized by RAFT polymerization was designed to resemble the antimicrobial peptide magainin (Mw: 2467 Da) and was generated from aminobutyl methacrylate and ethyl methacrylate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). The minimum inhibition concentration (MIC) value was found to be 15–20 μg mL−1 against S. aureus at pH 7.4. The MIC value was found to increase upon decreasing pH with an MIC value greater than 200 μg mL−1 as the pH reached 5.5. This was explained by decreasing pH leading to a diminishing of electrostatic interactions with the polymer (the bacteria become less “charged”) which results in reduced antibacterial activity. In addition, this polymer did not show any hemolytic activity or cytotoxicity to human dermal fibroblasts.
1 ratio). The minimum inhibition concentration (MIC) value was found to be 15–20 μg mL−1 against S. aureus at pH 7.4. The MIC value was found to increase upon decreasing pH with an MIC value greater than 200 μg mL−1 as the pH reached 5.5. This was explained by decreasing pH leading to a diminishing of electrostatic interactions with the polymer (the bacteria become less “charged”) which results in reduced antibacterial activity. In addition, this polymer did not show any hemolytic activity or cytotoxicity to human dermal fibroblasts.
Bansal et al.140 investigated n-butyl-modified linear polyethyleneimines (Mw: 25 kDa) against skin-based microbes from acne lesions. On five skin-isolated microbes (B. subtilis, S. aureus, S. typhimurium, K. pneumonia and E. coli) the polymers showed better antimicrobial activity than standard antibiotics (erythromycin, nadifloxacin, azelaic acid, and zinc monomethionine) which are generally used at 400–1200 μg mL−1. The polymers substituted with 20% levels of n-butyl groups showed the best action on skin isolated pathogens with MIC values between 130–200 μg mL−1 (linear polyethyleneimine had MIC values of 260–290 μg mL−1). Moreover, the 20% n-butyl substituted polymer inhibited the growth of S. epidermidis over 24 hours.
As an alternative class of antimicrobial polymers, “polyionenes” that contain quaternized ammonium groups along a backbone (in contrast to examples where quaternized ammonium groups are located as pendant groups) display potent antimicrobial properties against pathogens. In general, polyionenes can be synthesized either by step-growth polymerization of suitable monomers e.g., the Menshutkin reaction between alkyl dihalides and nucleophilic di/tertiary amines, self-polyaddition of aminoalkylhalides or cationic functionalization of precursor polymers.141,142 Liu et al.143 synthesized highly effective, skin compatible, cheap, and water-soluble polyionenes via commercially available monomers in a catalyst-free, polyaddition polymerization where the polymer-forming reaction and the installation of the charge occur simultaneously. The best polymer was prepared by reaction of N,N,N′,N′-tetramethyl ethylenediamine with di(chloromethyl)benzene and displayed excellent antimicrobial potency and the highest selectivity over mammalian cells compared to triclosan and chlorhexidine digluconate. The most active polymer inhibited the growth of bacteria between 2–31 mg mL−1, demonstrating strong broad spectrum antimicrobial activity against clinically isolated multidrug-resistant S. aureus, E. coli, P. aeruginosa, A. baumannii and K. pneumoniae, as well as fungi C. albicans and C. neoformans. In particular, a killing efficiency of more than 99.9% within 2 min was obtained. Dermal toxicity of the best polymer was evaluated with topical application of the polymer (200 and 500 μg mL−1) showing a better skin compatibility profile in mice compared to the clinically used surgical scrubs betadine and chlorhexidine. In addition, P. aeruginosa contaminated skin could be treated with the polymer with the number of bacteria on the skin significantly decreased compared to chlorhexidine handwash.
Alamri et al.144 prepared antimicrobial polymers from polyacrylonitrile that was functionalized with ethylenediamine and hexamethylenediamine (to give amidines). The amine side chains of the polymers were then functionalized with various benzaldehydes and the polymers were tested against S. aureus, P. aeruginosa, E. coli, S. typhi, A. flavus, A. niger, C. albicans, C. neoformans. All polymers showed antimicrobial activity with minimum inhibition concentrations as low as 12.5 μg mL−1 for polymers bearing long diamine linkers and 4-hydroxybenzaldehyde and 2,4-dihydroxybenzaldehyde side chains. Again, the mode of action relating to the positive charge of the polymers, specifically here the amidine functional group and presumably the free phenolic groups of the decorating benzaldehydes.
Polycarbonates are extensively used polymers due to their optical properties, heat resistance, and strength.145 Polycarbonates have been modified to allow quaternization and showed antibacterial activity.146 Thus Qiao et al.147 prepared a library of polycarbonates using a variety of cyclic carbonates (including one with pendent 3-chloropropyl groups) and diols via metal-free organocatalytic ring-opening polymerization. Subsequently the 3-chloropropyl groups were displaced with trimethyl amine to give cationic groups along the polymer chains. Although all polymers showed activity against S. aureus, the most pronounced activity was found for the polymer having 60% cationic charge (Mw; 16 kDa) with an MIC value of 63 μg mL−1. Field emission scanning electron microscopy images showed cellular lysis of E. coli and S. aureus.
Nimmagadda et al.148 reported amine containing polycarbonates that showed antibacterial activity synthesised using two cyclic carbonate monomers (one that contained a Boc-protected amine group that becomes hydrophilic upon deprotection and one hydrophobic) with random and diblock copolymers synthesized by ring opening polymerization. The most potent polymer, against three Gram-positive bacterial strains S. aureus, S. epidermidis and E. Faecalis, had MIC values between 1.6–5.0 μg mL−1, and consisted of approximately 20 monomers and formed micelles with a size of 228 nm. The mechanism of the polymer was explained as electrostatic interactions with the surface of bacteria and with free chains penetrating through the cell membrane leading to bacterial death.
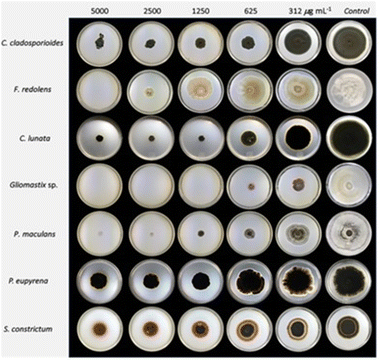 | ||
| Fig. 14 Inhibition of radial fungal growth observed using poly(2-(dimethylamino)ethyl methacrylate) quaternized with 1-iodobutane.136 Adapted from ref. 136 with permission from Elsevier. | ||
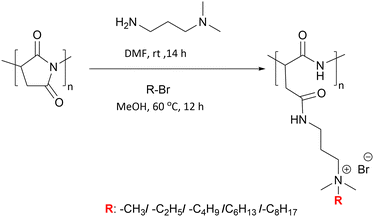 | ||
| Fig. 15 Synthesis route to the antimicrobial polymers based on a poly(aspartamide) backbone. Poly(succinimide) is ring opened with a primary amine (1-amino-3-dimethylaminopropane) and the tertiary amine subsequently converted to quaternary ammonium groups with different length alkyl chains.137 Reprinted the permission from {Yavvari, P. S.; Gupta, S.; Arora, D.; Nandicoori, V. K.; Srivastava, A.; Bajaj, A. Clathrin-Independent Killing of Intracellular Mycobacteria and Biofilm Disruptions Using Synthetic Antimicrobial Polymers. Biomacromolecules 2017, 18, 2024–2033} Copyright {2017} American Chemical Society. | ||
 | ||
| Fig. 16 SEM images of M. smegmatis (a) untreated and (b) upon the addition of the derivatised poly(aspartamide) (16 μg mL−1) after 9 hours. In this case, the polymer is quaternerized with methyl iodide. Scale bar = 200 nm.137 Reprinted the permission from {Yavvari, P. S.; Gupta, S.; Arora, D.; Nandicoori, V. K.; Srivastava, A.; Bajaj, A. Clathrin-Independent Killing of Intracellular Mycobacteria and Biofilm Disruptions Using Synthetic Antimicrobial Polymers. Biomacromolecules 2017, 18, 2024–2033} Copyright {2017} American Chemical Society. | ||
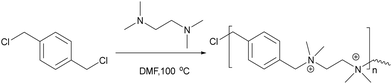 | ||
| Fig. 17 Synthesis of an antimicrobial “polyionene” via the polyaddition reaction of diamines and alkyl dihalides with both monomers readily varied.143 Reproduced from ref. 143 with permission from Elsevier. | ||
Poly(β-hydroxyl amine)s have been produced by polymerization between amines and bis-epoxy functionalities under aqueous conditions. These polymers can then be modified, post-polymerisation, to form quaternary derivatives. Oh et al.149 synthesized a polymer library of poly(β-hydroxyl amine) derivatives and investigated their antimicrobial activities with polymers bearing n-butyl quaternary amines showing the best activity against E. coli and S. aureus (MIC's <10–20 μg mL−1) (the best active polymer with two butyl chains on the quaternary ammonium groups) (see Fig. 18) also inhibited the growth of M. smegmatis at (20 μg mL−1). SEM images revealed significant damage to the bacterial membrane after polymer incubation.
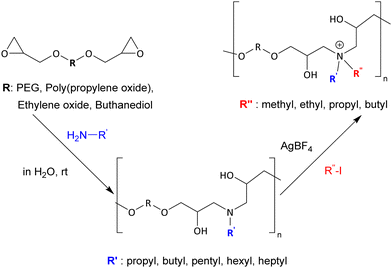 | ||
| Fig. 18 Synthesis route to a library of poly(β-hydroxyl amine)s by reacting diglycidyl monomers with various primary amines and subsequent quaternization with alkyl iodides.149 Reproduced from ref. 149 with permission from the Royal Society of Chemistry. | ||
Polyhexamethylene guanidine is a bactericidal agent that has been used in many applications such as antiseptics,150 water treatment,151 the food industry152 and personal hygiene applications.153 Zhuo et al.154 synthesized three oligoguanidine hydrochloride polymers bearing various alkyl chains e.g., butamethylene, hexamethylene and octanethylene and m-xylylene guanidine HCl by reacting, at high temperature, equimolar amounts of the appropriate diamine and guanidine hydrochloride. Synthesized oligomers were tested on 370 clinical strains with 96 of them antibiotic resistant. Among all the polymers, poly(octanethylene guanidine) hydrochloride showed the lowest minimum inhibition concentration (0.5–16 μg mL−1) in comparison to poly(hexamethylene guanidine) hydrochloride (1–64 μg mL−1) and the antiseptic and disinfectant, chlorhexidine digluconate (2–64 μg mL−1). Poly(octanethylene guanidine) hydrochloride was highly effective at 1–8 μg mL−1 against methicillin resistant S. aureus, vancomycin resistant E. faecium, multidrug resistant P. aeruginosa, ceftazidime resistant Citrobacter spp. and Enterobacter spp. The antimicrobial mechanism of the polymers was explained as the dual interaction of the hydrophobic alkyl chains of the polymers (anchoring into the membrane) and binding to the negatively charged phospholipids, causing damage to the bacterial membranes, although interactions with teichoic and lipoteichoic acids in the cell wall is also possible.
Studies have shown that the architecture of the polymers have a great impact on their antimicrobial efficiency.155–158 Thus Namivandi-Zangeneh et al.158 investigated the effect of amphiphilic ternary copolymers composed of oligoethylene glycol, cationic and hydrophobic functional groups with different chain lengths and topologies (i.e., random vs. block copolymers, and linear vs. hyperbranched polymers) on their antimicrobial activity and hemocompatibility. One of the hyperbranched random copolymers containing 2-ethylhexyl groups was found to have the best antimicrobial activity against P. aeruginosa and E. coli (MIC of 64 μg mL−1). Interestingly, polymers with different chain lengths (number-average degrees of polymerization of 100, 50 and 20) displayed similar antimicrobial activities but different hemolytic activities. For example, while shorter polymers caused hemolysis, hyperbranched polymers improved hemocompatibility (by >4 times) with only a minor loss in their antimicrobial activity. Among the hyperbranched polymers, hyperbranched random copolymers that contain 2-ethylhexyl groups were observed to have the best overall biocompatibility and the minimum inhibition concentration of the polymer was found to be 64 μg mL−1 against highly pathogenic Gram-negative species and 128 μg mL−1 against V. cholerae. Moreover, the most potent polymer (at a concentration of 64 μg mL−1) killed 99% of planktonic and 90% of biofilm bacteria, as well as inducing the dispersal of biofilms. Due to the cationic blocks in the polymer structure, antimicrobial modes of bactericidal action were observed for all polymer structures.
Santos et al.159 synthesized a library comprising homopolymers of (3-acrylamidopropyl)trimethylammonium chloride and amphiphilic linear, star-shaped (4 and 6-armed), random and block copolymers of hydrophilic (3-acrylamidopropyl) trimethylammonium chloride and hydrophobic n-butyl acrylate (the wide polymer library was generated using a “supplemental activator and reducing agent atom transfer radical polymerization”). The antimicrobial activity of polymers showed differences and in general amphiphilic polymers were more effective toward B. subtilis. Hydrophobicity changes in polymers’ composition showed no effect on the antimicrobial activity while the cationic segment of the polymer was necessary to generate antimicrobial activity. The antimicrobial activity of star-shaped polymers and linear polymers with similar molecular weights displayed similar antimicrobial activity, while enhanced antibacterial activities against Gram-positive and Gram-negative antimicrobial activity of polymer was observed with increasing molecular weight. The cationic homopolymer of poly(3-(acrylamidopropyl)trimethylammonium chloride) with the highest molecular weight and the 4-armed star shaped poly(3-(acrylamidopropyl)trimethylammonium chloride) showed the best activity against S. aureus, B. subtilis, B. cereus, E. coli and P. aeruginosa. Scanning electron microscopy and fluorescence microscopy showed that the cationic homopolymers and amphiphilic copolymers killed E. coli by disrupting the bacterial membrane.
It is accepted that polymer hydrophilic and hydrophobic balance is a key parameter in the design of antimicrobial polymers. Barman et al.160 explored the antimicrobial activity of cationic polymers modified with amino acids against drug resistant A. baumannii (one of the most notorious pathogens that causes hospital-derived (nosocomial) infections). In this context, cationic polymer precursors, poly(isobutylene-alt-N-(N′,N′-dimethylaminopropyl)maleimide) were synthesized by reacting poly(isobutylene-alt-maleic anhydride) with N,N-dimethyl-1,3-propanediamine. The resulting tertiary amine was reacted with esterified amino acids (glycine, L-alanine, D-alanine, L-valine, L-leucine, L-isoleucine, L-phenylalanine, L-tyrosine. L-Aspartic acid, L-glutamic acid) that had been acylated with bromoacetyl bromide to fully quaternerised the polymer (molecular weights were in the range of 17.4–20.8 kDa). The antimicrobial activity of the polymers was determined against both Gram-positive (S. aureus and E. faecium) and Gram-negative (E. coli and A. baumannii) bacteria, including methicillin resistant S. aureus, β-lactam-resistant K. pneumoniae, and several carbapenem-resistant A. baumannii. In general, the antimicrobial activity of the polymers was dependent on hydrophobicity variation originating from the amino acid in the polymer design (see Table 3) (however, with increasing hydrophobicity, the polymers showed toxicity against red blood cells). To illustrate, the glycine modified polymer had a minimum inhibition concentration value of 64 μg mL−1 for S. aureus and 8–16 μg mL−1 for A. baumannii, while the valine modified polymer showed minimum inhibition concentration values of 8–16 μg mL−1 for S. aureus, and 4 μg mL−1 against A. baumannii. The glycine modified polymer showed total killing (∼5 Log CFU mL−1 reduction) of the clinically isolated drug resistant A. baumannii after 1–2 hours at a concentration of 16 μg mL−1. This polymer not only was rapidly bactericidal against growing planktonic A. baumannii but also killed non-dividing stationary-phase bacteria instantaneously (<2 min). It is well-known that A. baumannii readily forms biofilms and the biofilm mass of drug-sensitive and drug-resistant A. baumannii were reduced by 65–85% at 64–128 μg mL−1 for the glycine-modified polymer. Importantly, there was no propensity for resistance development even after 14 passages with low levels of the polymer.
| Amino acid added | S. aureus | E. faecium | E. coli | A. baumannii | Drug resistant S. aureus | Drug resistant K. pneumoniae | Drug resistant A. baumannii |
|---|---|---|---|---|---|---|---|
| Gly | 64 | >128 | 32–64 | 8–16 | >128 | 128 | 8–16 |
| L-Ala | 64–128 | >128 | 32–64 | 8–16 | >128 | 128 | 8–16 |
| L-Val | 8–16 | 16 | 8 | 4 | 64 | 32 | 4 |
| L-Leu | 8 | 4–8 | 8 | 4 | 16 | 16–32 | 4 |
| L-Ile | 8 | 8 | 8 | 4 | 16 | 16 | 4 |
| L-Phe | 8 | 8 | 16 | 8 | 16 | 32 | 4–16 |
| L-Tyr | 16–32 | 64–128 | 32 | 32 | 32 | 64 | 16 |
| L-Asp | >128 | >128 | 64–128 | 16–32 | >128 | >128 | 16–32 |
| L-Glu | >128 | >128 | 64–128 | 16–32 | >128 | >128 | 16–32 |
Bactericidal polycationic polymers inactivate bacteria by disrupting the cellular envelope. In some cases, this is to electrostatic interactions with the “negatively charged bacteria cell membrane” and replacement of Ca2+ and/or Mg2+ by the polymeric biocidal cations. Clearly the type of counter anion will play an important role in the antimicrobial activity of quaternary ammonium and phosphonium group bearing antimicrobial polymers with the strength of the ion pair and solvation both important considerations. The Chauhan group studied the influence of counter ion on antimicrobial activity of polycations of poly(4-vinyl-2-hydroxyethyl pyridinium)161 and poly[1-vinyl-3-(2-sulfoethyl imidazolium betaine)]161 by exchanging Cl− or Br− with various OH−, SH−, NO3−, BF4− and CF3COO−. The polymer having hydroxy counter ions exhibited the strongest antifungal and antibacterial activity (minimum inhibition concentrations of 1040 and 520 μg mL−1 for M. circenelliods and A. niger and 650 μg mL−1 for B. coagulans). This phenomenon was explained by the enhanced the solubility of the polymers (Fig. 19–21).
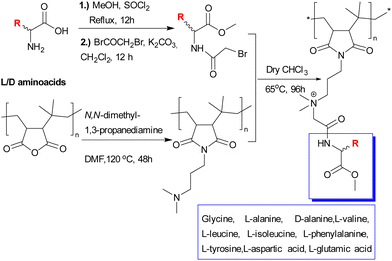 | ||
| Fig. 19 Synthesis of poly(isobutylene-alt-N-(N′,N′-dimethylaminopropyl)maleimide) by reacting poly(isobutylene-alt-maleic anhydride) with N,N-dimethyl-1,3-propanediamine and functionalization with L or D amino acids (reacted with bromoacetyl bromide).160 Reprinted the permission from {S. Barman, M. M. Konai, S. Samaddar and J. Haldar, Amino Acid Conjugated Polymers: Antibacterial Agents Effective against Drug-Resistant Acinetobacter baumannii with No Detectable Resistance, Appl. Mater., 2019, 11, 33559–33572} Copyright {2019} American Chemical Society. | ||
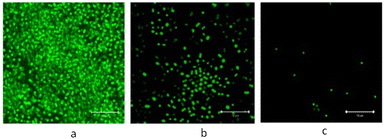 | ||
| Fig. 20 Confocal images of biofilm disruption of A. baumannii stained with crystal-violet after treatment with the glycine-modified polymers: (a) control (no polymer); (b) 64 μg mL−1 of the glycine-modified polymer; (c) 128 μg mL−1 of the glycine-modified polymer.160 Reprinted the permission from {S. Barman, M. M. Konai, S. Samaddar and J. Haldar, Amino Acid Conjugated Polymers: Antibacterial Agents Effective against Drug-Resistant Acinetobacter baumannii with No Detectable Resistance, Appl. Mater., 2019, 11, 33559–33572} Copyright {2019} American Chemical Society. | ||
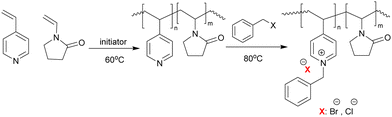 | ||
| Fig. 21 Synthesis of poly(4-vinylpyridine-co-N-vinylpyrrolidone) via RAFT polymerization and subsequent post-synthesis quaternization with benzyl halides.163 Reproduced from ref. 163 with permission from MDPI. | ||
The counter ion of poly[1-vinyl-3-(2-sulfoethyl imidazolium betaine)] was changed to Cl−, F−, OH−, SH−, SCN−, NO3−, BF4− and CH3COO−. In terms of Gram-positive bacteria (B. coagulans) again the hydroxyl counter ions polymers showed the strongest inhibition of the growth of bacteria. In contrast, for Gram-negative bacteria (P. aeruginosa), the F−, SH− and NO3− counter ions were the most effective. Regarding the inhibition of the growth of fungi, SH− displayed maximal activity against M. circenelliods while OH− was found the most effective against B. fulva. It was suggested that the identity of counter anion has an effect on the efficiency and selectivity towards individual microbes due to the discrepancy of polymer morphology and the solubility of polycations in water, resulting in different antimicrobial performance.162 However, how these anions are related/exchanged with the ions in the buffers and media, and the pH of the applied polymers, are important considerations and are often poorly discussed.
Insoluble pyridinium-containing polymers can capture bacteria intact, while soluble pyridinium polymers can kill bacteria by interacting with, and disrupting, the bacterial cell membrane/wall. Xue et al.163 synthesized water-soluble pyridinium-type copolymers that possessed both antibacterial and antiviral activities. To overcome cytotoxicity and solubility issues N-vinyl pyrrolidone was chosen as one of the monomers (along with vinyl pyridine), with the pyridine-based polymers subsequently quaternized with benzyl halides. Various monomer feed ratios were used to optimize the antimicrobial activity. Both homopolymers and copolymers of pyridinium-type polymers (Mw ∼ 90–100 kDa) were alkylated and the charge density of the quaternary pyridinium polymers was calculated by colloidal titration. The MIC values of the polymers decreased with an increase in positive charge density, while polymers quaternized with benzyl bromide showing the most potent antimicrobial activity against Gram-negative bacteria (E. coli) compared to the ones quaternized with benzyl chloride. This was explained by authors as due to the change in the dissociation ability of the polymers being dependent on the counter ion type. Ito suggested the mode of action of the polymers could be explained as a change in the mechanism related to the displacement of calcium and/or magnesium ions on the cell membrane. If an ammonium ion forms a tight ion-pair with its counter anion it may delay the ionic dissociation of the quaternary ammonium salt, influencing the displacement of those divalent ions. This may explain why benzyl bromide quaternized polymers showed higher antibacterial activity than the ones with benzyl chloride. However, other rational may presumably be due to the reduced levels of alkylation with benzyl chloride vs. the bromide. Analysis by AFM showed that the polymers were able to kill E. coli within 3 min by disrupting the cellular envelope with leakage of intracellular components. Enveloped virus e.g. influenza or herpes are also protected by a lipid membrane21 and quaternized ammonium cationic polymers can also effectively kill some viruses. In this context, pyridine-based polymers quaternerised with benzyl halides and showed virucidal activity, killing 95% of influenza virus at 50 ppm. The virucidal action of the polymers was dependant on interactions between the lipid envelope and the polymers, causing disorganization and severe damage to the lipid envelope.
3. Conclusions and future perspective
Microbial-based diseases and contamination are still a major burden on the economies and health care systems of countries around the world. Moreover, microorganisms have gained resistance against existing drugs over the decades due to misuse or overuse – although it should be noted that this is not a recent phenomenon – with resistance against penicillin identified in the 1940's. New alternatives have been sought to solve these life-threatening problems. In the light of successful studies and antimicrobial polymer-based materials are becoming a tool in the arsenal to fight against pathogens, indeed as high-lighted in this review polymeric-based materials were used as surface coatings to destroy SARS-COVID-2. Importantly, since most antibacterial polymers disrupt the cell envelope of bacteria the chance of resistance formation by the pathogens against polymers is unlikely in contrast to conventional antibiotics which are largely specific/single target based. The long-term vision is that antimicrobial polymers with negligible toxicity, could become an additional option to current antibiotics. Clearly as is already happening antimicrobial polymer-based materials are becoming ever more part of our daily lives. Going forwards it is key that multi-drug resistant and clinically isolated pathogens are included much more in studies to enlighten the mechanisms of action of antimicrobial polymers and to broaden their structural scope and application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank Dr Daniel Norman and Dr Matthew Owens for their support and ideas during writing this review. This work is funded by an award – a PhD scholarship by Turkish Ministry of National Education Study Abroad Programme.References
- A. Muñoz-Bonilla and M. Fernández-García, Prog. Polym. Sci., 2012, 37, 281–339 CrossRef.
- K. S. Huang, C. H. Yang, S. L. Huang, C. Y. Chen, Y. Y. Lu and Y. S. Lin, Int. J. Mol. Sci., 2016, 17, 1578 CrossRef PubMed.
- A. Kyzioł, W. Khan, V. Sebastian and K. Kyzioł, Chem. Eng. J., 2020, 385, 123888 CrossRef.
- B. S. T. Peddinti, S. N. Downs, J. Yan, S. D. Smith, R. A. Ghiladi, V. Mhetar, R. Tocchetto, A. Griffiths, F. Scholle and R. J. Spontak, Adv. Sci., 2021, 8, 1–9 Search PubMed.
- R. J. Cornell and L. G. Donaruma, J. Med. Chem., 1965, 8, 388–390 CrossRef CAS PubMed.
- E. F. Palermo and K. Kuroda, Appl. Microbiol. Biotechnol., 2010, 87, 1605–1615 CrossRef CAS PubMed.
- O. Vogl and D. Tirrell, J. Macromol. Sci., Part A: Pure Appl. Chem., 1979, A13, 37–41 Search PubMed.
- C. R. Allan and L. A. Hadwiger, Exp. Mycol., 1979, 3, 285–287 CrossRef CAS.
- S. Shima, H. Matsuoka, T. Iwamoto and H. Sakai, J. Antibiot., 1984, 37, 1449–1455 CrossRef CAS PubMed.
- T. S. Ikeda Tomiki, Makromol. Chem., 1984, 185, 869–876 CrossRef.
- G. Pasparakis and C. Alexander, Analyst, 2007, 132, 1075–1082 RSC.
- M. Charnley, M. Textor and C. Acikgoz, React. Funct. Polym., 2011, 71, 329–334 CrossRef CAS.
- F. Siedenbiedel and J. C. Tiller, Polymers, 2012, 4, 46–71 CrossRef CAS.
- A. Jain, L. S. Duvvuri, S. Farah, N. Beyth, A. J. Domb and W. Khan, Adv. Healthcare Mater., 2014, 3, 1969–1985 CrossRef CAS PubMed.
- A. Arora and A. Mishra, Mater. Today Proc., 2018, 5, 17156–17161 CrossRef CAS.
- N. F. Kamaruzzaman, L. P. Tan, R. H. Hamdan, S. S. Choong, W. K. Wong, A. J. Gibson, A. Chivu and M. De Fatima Pina, Int. J. Mol. Sci., 2019, 20, 1–31 Search PubMed.
- M. Liu, J. Li and B. Li, Langmuir, 2018, 34, 1574–1580 CrossRef CAS PubMed.
- P. Teratanatorn, R. Hoskins, T. Swift, C. W. I. Douglas, J. Shepherd and S. Rimmer, Biomacromolecules, 2017, 18, 2887–2899 CrossRef CAS PubMed.
- A. C. Engler, A. Shukla, S. Puranam, H. G. Buss, N. Jreige and P. T. Hammond, Biomacromolecules, 2011, 15, 1666–1674 CrossRef PubMed.
- M. J. Catalão, S. R. Filipe and M. Pimentel, Front. Microbiol., 2019, 10, 1–11 CrossRef PubMed.
- G. Xu, D. Pranantyo, L. Xu, K. Neoh, E. Kang and S. L. Teo, Ind. Eng. Chem. Res., 2016, 55, 2–11 Search PubMed.
- A. Kausar, J. Macromol. Sci., Part A: Pure Appl. Chem., 2018, 55, 440–448 CrossRef CAS.
- J. Hernandez-Montelongo, E. G. Lucchesi, V. F. Nascimento, C. G. França, I. Gonzalez, W. A. A. Macedo, D. Machado, M. Lancellotti, A. M. Moraes, M. M. Beppu and M. A. Cotta, Mater. Sci. Eng., C, 2017, 71, 718–724 CrossRef CAS.
- I. A. MacDonald and M. J. Kuehna, J. Bacteriol., 2013, 195, 2971–2981 CrossRef CAS PubMed.
- WHO/UNICEF Joint Monitoring Programme and S. and H. for Water Supply, Five years into the SDGs progress on household drinking water, sanitation and hygiene, 2020 Search PubMed.
- T. R. Sinclair, D. Robles, B. Raza, S. van den Hengel, S. A. Rutjes, A. M. de Roda Husman, J. de Grooth, W. M. de Vos and H. D. W. Roesink, Colloids Surf., A, 2018, 551, 33–41 CrossRef CAS.
- A. Nagaraja, Y. M. Puttaiahgowda, A. Kulal, A. M. Parambil and T. Varadavenkatesan, Macromol. Res., 2019, 27, 301–309 CrossRef CAS.
- E. H. Westman, M. Ek, L. E. Enarsson and L. Wågberg, Biomacromolecules, 2009, 10, 1478–1483 CrossRef CAS.
- S. Pernagallo, M. Wu, P. Gallagher and M. Bradley, J. Mater. Chem., 2011, 21, 96–101 RSC.
- H. Pickering, M. Wu, M. Bradley and H. Bridle, Environ. Sci. Technol., 2012, 46, 2179–2186 CrossRef CAS.
- S. Venkateswaran, M. Wu, P. J. Gwynne, A. Hardman, A. Lilienkampf, S. Pernagallo, G. Blakely, D. G. Swann, M. P. Gallagher and M. Bradley, J. Mater. Chem. B, 2014, 2, 6723–6729 RSC.
- A. L. Hook, C. Chang, J. Yang, J. Luckett, A. Cockayne, S. Atkinson, Y. Mei, R. Bayston, D. J. Irvine, R. Langer, D. G. Anderson, P. Williams, M. C. Davies and M. R. Alexander, Nat. Biotechnol., 2012, 30, 868–875 CrossRef CAS PubMed.
- A. L. Hook, C. Y. Chang, J. Yang, S. Atkinson, R. Langer, D. G. Anderson, M. C. Davies, P. Williams and M. R. Alexander, Adv. Mater., 2013, 25, 2542–2547 CrossRef CAS.
- A. A. Dundas, O. Sanni, J. Dubern, G. Dimitrakis, A. L. Hook, D. J. Irvine, P. Williams and M. R. Alexander, Adv. Mater., 2019, 31, 1903513 CrossRef CAS PubMed.
- K. Adlington, N. T. Nguyen, E. Eaves, J. Yang, C. Chang, J. Li, A. L. Gower, A. Stimpson, D. G. Anderson, R. Langer, M. C. Davies, A. L. Hook, P. Williams, M. R. Alexander and D. J. Irvine, Biomacromolecules, 2016, 17, 2830–2838 CrossRef CAS PubMed.
- S. R. J. Peddinti Bharadwaja, S. T. Scholle Frank, G. Vargas Mariana, D. Smith Steven and A. Ghiladi Reza, Mater. Horiz., 2019, 6, 2056–2062 RSC.
- H. M. Minhas, A. Scheel, P. Garibaldi and B. Liu, Lancet Infect. Dis., 2020, 892–893 Search PubMed.
- M. Jayaweera, H. Perera, B. Gunawardana and J. Manatunge, Environ. Res., 2020, 188, 1–18 CrossRef PubMed.
- H. Keum, D. Kim, C.-H. Whang, A. Kang, S. Lee, W. Na and S. Jon, ACS Omega, 2022, 7, 10526–10538 CrossRef CAS PubMed.
- L. Q. Xu, D. Pranantyo, K. Neoh, E. Kang, S. L. Teo and G. D. Fu, Polym. Chem., 2015, 7, 493–501 RSC.
- L. Han, L. Yan, K. Wang, L. Fang, H. Zhang, Y. Tang, Y. Ding, L. Weng, J. Xu, J. Weng, Y. Liu, F. Ren and X. Lu, Nat. Publ. Gr., 2017, e372 CAS.
- F. Gao, H. Qu, Y. Duan, J. Wang, X. Song and T. Ji, RSC Adv., 2014, 6657–6663 RSC.
- S. Kumar, M. Perikamana, J. Lee, Y. Bin Lee, Y. M. Shin, E. J. Lee, A. G. Mikos and H. Shin, Biomacromolecules, 2015, 16, 2541–2555 CrossRef PubMed.
- X. Wang, S. Jing, Y. Liu, S. Liu and Y. Tan, Polymer, 2017, 116, 314–323 CrossRef CAS.
- L. Luo, G. Li, D. Luan, Q. Yuan, Y. Wei and X. Wang, Appl. Mater., 2014, 6, 19371–19377 CrossRef CAS PubMed.
- J. Xu, Y. Bai, M. Wan, Y. Liu, L. Tao and X. Wang, Polymers, 2018, 10, 1–12 Search PubMed.
- E. A. Chamsaz, S. Mankoci, H. A. Barton and A. Joy, Appl. Mater., 2017, 9, 6704–6711 CrossRef CAS.
- J. Hoque, P. Akkapeddi, V. Yadav, G. B. Manjunath, D. S. S. M. Uppu, M. M. Konai, V. Yarlagadda, K. Sanyal and J. Haldar, ACS Appl. Mater. Interfaces, 2015, 7, 1804–1815 CrossRef CAS PubMed.
- J. Hoque, P. Akkapeddi, C. Ghosh, D. S. S. M. Uppu and J. Haldar, Appl. Mater., 2016, 8, 29298–29309 CrossRef CAS PubMed.
- A. Tabriz, M. A. Ur Rehman Alvi, M. B. Khan Niazi, M. Batool, M. F. Bhatti, A. L. Khan, A. U. Khan, T. Jamil and N. M. Ahmad, Carbohydr. Polym., 2019, 207, 17–25 CrossRef CAS.
- U. Ajdnik, M. Finšgar and L. Fras Zemljič, Carbohydr. Polym., 2020, 232, 115817 CrossRef CAS PubMed.
- A. A. Sharipova, S. B. Aidarova, D. Grigoriev, B. Mutalieva, G. Madibekova, A. Tleuova and R. Miller, Colloids Surf., B, 2016, 137, 152–157 CrossRef CAS.
- H. Yu, L. Liu, X. Li, R. Zhou, S. Yan, C. Li and S. Luan, Chem. Eng. J., 2019, 360, 1030–1041 CrossRef CAS.
- S. Dhingra, A. Joshi, N. Singh and S. Saha, Mater. Sci. Eng., C, 2021, 118, 111465 CrossRef CAS PubMed.
- F. Aynali, E. Doganci, T. Doruk and H. Sadikoglu, Polym. Int., 2019, 68, 385–393 CrossRef CAS.
- F. Aynali, H. Balci, E. Doganci and E. Bulus, Eur. Polym. J., 2021, 149, 7–8 CrossRef.
- P. Varguez-Catzim, N. Rodríguez-Fuentes, R. Borges-Argáez, M. Cáceres-Farfán, A. González-Díaz, A. Alonzo-Garcia, S. Duarte, M. Aguilar-Vega and M. O. González-Díaz, Appl. Surf. Sci., 2021, 565, 150544 CrossRef CAS.
- J. Lv and Y. Cheng, Chem. Soc. Rev., 2021, 50, 5435–5467 RSC.
- D. J. Han, S. Kim, H. J. Heo, C. Jin, J. Young Kim, H. Choi, I. J. Park, H. S. Kang, S. G. Lee, J. C. Lee and E. H. Sohn, Appl. Surf. Sci., 2021, 562, 150181 CrossRef CAS.
- M. D. Macià, E. Rojo-Molinero and A. Oliver, Clin. Microbiol. Infect., 2014, 20, 981–990 CrossRef PubMed.
- K. Zhang, X. Li, C. Yu and Y. Wang, Front. Cell. Infect. Microbiol., 2020, 10, 1–16 CrossRef PubMed.
- A. Vishwakarma, F. Dang, A. Ferrell, H. A. Barton and A. Joy, JACS, 2021, 143, 9440–9449 CrossRef CAS.
- L. Zhai, Chem. Soc. Rev., 2013, 42, 7148–7160 RSC.
- I. Erel-unal and S. A. Sukhishvili, Macromolecules, 2008, 41, 3962–3970 CrossRef CAS.
- C. B. Amphiphiles, Ber. Bunsetlges. Phys. Chem., 1991, 5, 1430–1434 Search PubMed.
- X. Zhu and X. Jun Loh, Biomater. Sci., 2015, 3, 1505–1518 RSC.
- J. A. Lichter and M. F. Rubner, Langmuir, 2009, 25, 7686–7694 CrossRef CAS PubMed.
- E. Wulandari, R. Namivandi-zangeneh, P. R. Judzewitsch, R. Budhisatria, A. H. Soeriyadi, C. Boyer and E. H. H. Wong, ACS Appl. Mater. Interfaces, 2021, 4, 692–700 CrossRef CAS.
- H. Khalil, T. Chen, R. Riffon, R. Wang and Z. Wang, Antimicrob. Agents Chemother., 2008, 52, 1635–1641 CrossRef CAS PubMed.
- J. Lin, S. Qiu, K. Lewis and A. M. Klibanov, Biotechnol. Prog., 2002, 18, 1082–1086 CrossRef CAS.
- S. Y. Wong, Q. Li, J. Veselinovic, B. S. Kim, A. M. Klibanov and P. T. Hammond, Biomaterials, 2010, 31, 4079–4087 CrossRef CAS PubMed.
- F. Wang, Z. Yao, H. Wu, S. Zhang, N. Zhu and X. Gai, Appl. Mech. Mater., 2012, 108, 194–199 CAS.
- A. V. Briones, T. Sato and U. G. Bigol, Adv. Chem. Eng. Sci., 2014, 04, 233–241 CrossRef.
- E. I. Rabea, M. E. T. Badawy, C. V. Stevens, G. Smagghe and W. Steurbaut, Biomacromolecules, 2003, 4, 1457–1465 CrossRef CAS PubMed.
- M. Kumorek, I. M. Minisy, T. Krunclová, M. Voršiláková, K. Venclíková, E. M. Chánová, O. Janoušková and D. Kubies, Mater. Sci. Eng., C, 2020, 109, 111049 CrossRef PubMed.
- J. Hernández-Montelongo, V. F. Nascimento, D. Murillo, T. B. Taketa, P. Sahoo, A. A. De Souza, M. M. Beppu and M. A. Cotta, Carbohydr. Polym., 2016, 136, 1–11 CrossRef PubMed.
- Y. Zhang, F. Wang, Q. Huang, A. B. Patil, J. Hu, L. Fan, Y. Yang, H. Duan, X. Dong and C. Lin, Mater. Sci. Eng., C, 2020, 110, 110690 CrossRef CAS.
- J. Illergård, L. Wågberg and M. Ek, Colloids Surf., B, 2011, 88, 115–120 CrossRef PubMed.
- J. Illergård, U. Römling, L. Wågberg and M. Ek, Cellulose, 2012, 19, 1731–1741 CrossRef.
- M. Illergård, J. Wågberg and L. Ek, Cellulose, 2015, 22, 2023–2034 CrossRef.
- C. Chen and M. Ek, Nord. Pulp Pap. Res. J., 2018, 33, 385–396 CrossRef CAS.
- C. Chen, J. Illergård, L. Wågberg and M. Ek, Holzforschung, 2017, 71, 649–658 CAS.
- H. Schmolke, S. Demming, A. Edlich, V. Magdanz, S. Büttgenbach, E. Franco-Lara, R. Krull and C.-P. Klages, Biomicrofluidics, 2010, 4, 044113 CrossRef PubMed.
- Y. Zhu, X. Yu, T. Zhang and X. Wang, Appl. Surf. Sci., 2019, 483, 979–990 CrossRef CAS.
- L. Tang, W. Gu, P. Yi, J. L. Bitter, J. Yeon, D. H. Fairbrother and K. Loon, J. Membr. Sci., 2013, 446, 201–211 CrossRef CAS.
- I. C. Gîfu, M. E. Maxim, L. O. Cinteza, M. Popa, L. Aricov, A. R. Leonties, M. Anastasescu, D. F. Anghel, R. Ianchis, C. M. Ninciuleanu, S. G. Burlacu, C. L. Nistor and C. Petcu, Coatings, 2019, 9, 1–13 CrossRef.
- E. N. Gibbons, C. Winder, E. Barron, D. Fernandes, M. J. Krysmann, A. Kelarakis, A. V. S. Parry and S. G. Yeates, Nanomaterials, 2019, 9, 1563 CrossRef CAS PubMed.
- A. F. Martins, J. Vlcek, T. Wigmosta, M. Hedayati, M. M. Reynolds, K. C. Popat and M. J. Kipper, Appl. Surf. Sci., 2020, 502, 144282 CrossRef CAS.
- S. P. Facchi, A. C. de Oliveira, E. O. T. Bezerra, J. Vlcek, M. Hedayati, M. M. Reynolds, M. J. Kipper and A. F. Martins, Eur. Polym. J., 2020, 130, 109677 CrossRef CAS.
- S. Del Hoyo-Gallego, L. Pérez-Álvarez, F. Gómez-Galván, E. Lizundia, I. Kuritka, V. Sedlarik, J. M. Laza and J. L. Vila-Vilela, Carbohydr. Polym., 2016, 143, 35–43 CrossRef CAS PubMed.
- Y. Cho, M. Lee, S. Park, Y. Kim, E. Lee and S. G. Im, Biotechnol. Bioprocess Eng., 2021, 178, 165–178 CrossRef PubMed.
- C. Su, Y. Hu, Q. Song, Y. Ye, L. Gao, P. Li and T. Ye, Appl. Biochem. Biotechnol., 2020, 12, 18978–18986 CAS.
- Q. Song, R. Zhao, T. Liu, L. Gao, C. Su, Y. Ye, S. Y. Chan, X. Liu, K. Wang, P. Li and W. Huang, Chem. Eng. J., 2021, 418, 1–10 Search PubMed.
- N. Hadjesfandiari, K. Yu, Y. Mei and J. N. Kizhakkedathu, J. Mater. Chem. B, 2014, 2, 4968–4978 RSC.
- R. Mohammadi Sejoubsari, A. P. Martinez, Y. Kutes, Z. Wang, A. V. Dobrynin and D. H. Adamson, Macromolecules, 2016, 49, 2477–2483 CrossRef CAS.
- B. Zdyrko and I. Luzinov, Macromol. Rapid Commun., 2011, 32, 859–869 CrossRef CAS PubMed.
- J. Peng, P. Liu, W. Peng, J. Sun, X. Dong, Z. Ma, D. Gan, P. Liu and J. Shen, J. Hazard. Mater., 2021, 411, 125110 CrossRef CAS PubMed.
- T. Çaykara, M. G. Sande, N. Azoia, L. R. Rodrigues, C. Joana and C. J. Silva, Med. Microbiol. Immunol., 2020, 209, 363–372 CrossRef PubMed.
- W. Cao, D. Wei, Y. Jiang, S. Ye, A. Zheng and Y. Guan, J. Mater. Sci., 2019, 54, 2699–2711 CrossRef CAS.
- D. D. Larikov, M. Kargar, A. Sahari, L. Russel, K. T. Gause, B. Behkam and W. A. Ducker, Biomacromolecules, 2014, 15, 169–176 CrossRef PubMed.
- M. Li, D. Mitra, E. T. Kang, T. Lau, E. Chiong and K. G. Neoh, ACS Appl. Mater. Interfaces, 2017, 9, 1847–1857 CrossRef CAS.
- V. G. Correia, A. M. Ferraria, M. G. Pinho and A. Aguiar-Ricardo, Biomacromolecules, 2015, 16, 3904–3915 CrossRef CAS PubMed.
- E. Koufakis, T. Manouras, S. H. Anastasiadis and M. Vamvakaki, Langmuir, 2020, 36, 3482–3493 CrossRef CAS PubMed.
- W. Yandi, S. Mieszkin, M. E. Callow, J. A. Callow, J. A. Finlay, B. Liedberg and T. Ederth, Biofouling, 2017, 33, 169–183 CrossRef CAS PubMed.
- S. Lu, Z. Tang, W. Li, X. Ouyang, S. Cao, L. Chen, L. Huang, H. Wu and Y. Ni, Cellulose, 2018, 25, 7261–7275 CrossRef CAS.
- F. Alipour, S. Khaheshi, M. Soleimanzadeh, S. Heidarzadeh and F. E. Hospital, J. Ophthalmic Vision Res., 2017, 2, 193–204 Search PubMed.
- S. Kumar, R. Pillai, S. Reghu, Y. Vikhe, H. Zheng, C. H. Koh and M. B. Chan-park, Macromol. Rapid Commun., 2020, 41, 20000175 Search PubMed.
- G. Gao, K. Yu, J. Kindrachuk, D. E. Brooks, R. E. W. Hancock and J. N. Kizhakkedathu, Biomacromolecules, 2011, 12, 3715–3727 CrossRef CAS.
- G. Gao, D. Lange, K. Hilpert, J. Kindrachuk, Y. Zou, J. T. J. Cheng, M. Kazemzadeh-Narbat, K. Yu, R. Wang, S. K. Straus, D. E. Brooks, B. H. Chew, R. E. W. Hancock and J. N. Kizhakkedathu, Biomaterials, 2011, 32, 3899–3909 CrossRef CAS PubMed.
- M. Gultekinoglu, Y. Tunc, C. Erdogdu, M. Sagiroglu, E. Ayse, Y. Jin, P. Hinterdorfer and K. Ulubayram, Acta Biomater., 2015, 21, 44–54 CrossRef CAS PubMed.
- M. Gultekinoglu, S. Karahan, D. Kart, M. Sagiroglu, N. Erta, A. H. Ozen and K. Ulubayram, Mater. Sci. Eng., C, 2017, 71, 1166–1174 CrossRef CAS PubMed.
- S. Krishnan, C. J. Weinman and C. K. Ober, J. Mater. Chem., 2008, 18, 3405–3413 RSC.
- D. Leckband, S. Sheth and A. Halperin, J. Biomater. Sci., Polym. Ed., 1999, 10, 1125–1147 CrossRef CAS PubMed.
- S. Chen, L. Li, C. Zhao and J. Zheng, Polymer, 2010, 51, 5283–5293 CrossRef CAS.
- W. L. Chen, R. Cordero, H. Tran and C. K. Ober, Macromolecules, 2017, 50, 4089–4113 CrossRef CAS.
- X. Yu, Y. Yang, W. Yang, X. Wang, X. Liu, F. Zhou and Y. Zhao, J. Colloid Interface Sci., 2022, 610, 234–245 CrossRef CAS PubMed.
- X. Y. Zhang, Y. Q. Zhao, Y. Zhang, A. Wang, X. Ding, Y. Li, S. Duan, X. Ding and F. J. Xu, Biomacromolecules, 2019, 20, 4171–4179 CrossRef CAS PubMed.
- M. Hoyos-Nogués, J. Buxadera-Palomero, M. P. Ginebra, J. M. Manero, F. J. Gil and C. Mas-Moruno, Colloids Surf., B, 2018, 169, 30–40 CrossRef.
- Y. Fu, Y. Yang, S. Xiao, L. Zhang, L. Huang, F. Chen, P. Fan, M. Zhong, J. Tan and J. Yang, Prog. Org. Coat., 2019, 130, 75–82 CrossRef CAS.
- Y. He, X. Wan, K. Xiao, W. Lin, J. Li, Z. Li, F. Luo, H. Tan, J. Li and Q. Fu, Biomater. Sci., 2019, 7, 5369–5382 RSC.
- Y. Zhang, X. Zhang, Y. Q. Zhao, X. Y. Zhang, X. Ding, X. Ding, B. Yu, S. Duan and F. J. Xu, Biomater. Sci., 2020, 8, 997–1006 RSC.
- G. Xu, X. Liu, P. Liu, D. Pranantyo, K. Neoh and E. Kang, Langmuir, 2017, 33, 6926–6936 Search PubMed.
- G. Xu, K. G. Neoh, E. T. Kang and S. L. M. Teo, ACS Sustainable Chem. Eng., 2020, 8, 2586–2595 CrossRef CAS.
- X. Xiong, Z. Wu, Q. Yu, L. Xue, J. Du and H. Chen, Langmuir, 2015, 31, 12054–12060 CrossRef CAS.
- S. Yan, S. Luan, H. Shi, X. Xu, J. Zhang, S. Yuan, Y. Yang and J. Yin, Biomacromolecules, 2016, 17, 1696–1704 CrossRef CAS PubMed.
- T. Chen, L. Zhao, Z. Wang, J. Zhao, Y. Li, H. Long, D. Yu and X. Wu, Biomacromolecules, 2020, 21, 5213–5221 CrossRef CAS.
- M. A. Rahman, M. Bam, E. Luat, M. S. Jui, M. S. Ganewatta, T. Shokfai, M. Nagarkatti, A. W. Decho and C. Tang, Nat. Commun., 2018, 9, 1–10 CrossRef PubMed.
- M. A. Rahman, M. S. Jui, M. Bam, Y. Cha, E. Luat, A. Alabresm, M. Nagarkatti, A. W. Decho and C. Tang, ACS Appl. Mater. Interfaces, 2020, 12, 21221–21230 CrossRef CAS PubMed.
- M. Zhou, Y. Qian, J. Xie, W. Zhang, W. Jiang, X. Xiao, S. Chen, C. Dai, Z. Cong, Z. Ji, N. Shao, L. Liu, Y. Wu and R. Liu, Angew. Chemie, 2020, 132, 6474–6481 CrossRef.
- V. A. B. Mayandi Venkatesh, E. T. L. Goh, A. J. Y. Raditya Anggara, M. Hussain Urf Turabe Fazil, S. L. Sriram Harini, T. Tun Aung, A. Stephen John Fox, L. Yang, X. J. L. Timothy Mark Sebastian Barkham, R. W. B. Navin Kumar Verma and R. Lakshminarayanana, Antimicrob. Agents Chemother., 2017, 61, 1–15 Search PubMed.
- Z. Tan, Y. Shi, B. Xing, Y. Hou, J. Cui and S. Jia, Bioresour. Bioprocess., 2019, 6, 1–10 CrossRef.
- Z. Su, Q. Han, F. Zhang, X. Meng and B. Liu, Carbohydr. Polym., 2020, 230, 115635 CrossRef CAS.
- W. Y. Ding, S. Di Zheng, Y. Qin, F. Yu, J. W. Bai, W. Q. Cui, T. Yu, X. R. Chen, G. Bello-Onaghise and Y. H. Li, Front. Chem., 2019, 7, 1–14 CrossRef PubMed.
- D. J. Phillips, J. Harrison, S. J. Richards, D. E. Mitchell, E. Tichauer, A. T. M. Hubbard, C. Guy, I. Hands-Portman, E. Fullam and M. I. Gibson, Biomacromolecules, 2017, 18, 1592–1599 CrossRef CAS PubMed.
- L. M. Thoma, B. R. Boles and K. Kuroda, Biomacromolecules, 2014, 2933–2943 CrossRef CAS.
- M. A. De Jesús-Tellez, S. De Rosa-garcía, I. Medrano-galindo, I. Rosales-pe, U. S. Schubert and P. Quintana-owen, React. Funct. Polym., 2021, 163, 104887 CrossRef.
- P. S. Yavvari, S. Gupta, D. Arora, V. K. Nandicoori, A. Srivastava and A. Bajaj, Biomacromolecules, 2017, 18, 2024–2033 CrossRef CAS PubMed.
- S. Mukherjee, S. Barman, R. Mukherjee and J. Haldar, Front. Bioeng. Biotechnol., 2020, 8, 1–19 CrossRef PubMed.
- S. Hong, H. Takahashi, E. T. Nadres, H. Mortazavian, G. A. Caputo, J. G. Younger and K. Kuroda, PLoS One, 2017, 12, 1–17 CAS.
- R. Bansal, R. Pathak, B. Kumar, H. K. Gautam and P. Kumar, Colloid Polym. Sci., 2017, 295, 1177–1185 CrossRef CAS.
- Y. Xue, H. Xiao and Y. Zhang, Int. J. Mol. Sci., 2015, 16, 3626–3655 CrossRef CAS PubMed.
- S. R. Williams and T. E. Long, Prog. Polym. Sci., 2009, 34, 762–782 CrossRef CAS.
- S. Liu, R. J. Ono, H. Wu, J. Yng, Z. Chang, K. Xu, M. Zhang, G. Zhong, J. P. K. Tan, M. Ng, C. Yang, J. Chan, Z. Ji, C. Bao, K. Kumar, S. Gao, A. Lee, M. Fevre, H. Dong, J. Y. Ying, L. Li, W. Fan, J. L. Hedrick and Y. Yan, Biomaterials, 2017, 127, 36–48 CrossRef CAS PubMed.
- A. Alamri, M. H. El-Newehy and S. S. Al-Deyab, Chem. Cent. J., 2012, 6, 1–13 CrossRef PubMed.
- S. P. Rogalsky, O. V. Moshynets, L. G. Lyoshina and O. P. Tarasyuk, EPMA J., 2014, 5, A133 CrossRef.
- V. Wee, L. Ng, J. Pang, K. Tan, J. Leong, Z. X. Voo and J. L. Hedrick, Macromolecules, 2014, 47, 1285–1291 CrossRef.
- Y. Qiao, C. Yang, D. J. Coady, Z. Y. Ong, J. L. Hedrick and Y. Y. Yang, Biomaterials, 2012, 33, 1146–1153 CrossRef CAS PubMed.
- A. Nimmagadda, X. Liu, P. Teng, M. Su, Y. Li, Q. Qiao, N. K. Khadka, X. Sun, J. Pan, H. Xu, Q. Li and J. Cai, Biomacromolecules, 2017, 18, 87–95 CrossRef CAS PubMed.
- J. Oh, S. Kim, M. Oh and A. Khan, RSC Adv., 2020, 10, 26752–26755 RSC.
- F. G. G. Dias, L. de, F. Pereira, R. L. T. Parreira, R. C. S. Veneziani, T. C. Bianchi, V. F. N. de, P. Fontes, M. de, C. Galvani, D. D. P. Cerce, C. H. G. Martins, F. Rinaldi-Neto, N. H. Ferreira, L. H. D. da Silva, L. T. S. de Oliveira, T. R. Esperandim, F. A. de Sousa, S. R. Ambrósio and D. C. Tavares, Eur. J. Pharm. Sci., 2021, 160, 105739 CrossRef CAS PubMed.
- I. J. Asiedu-Gyekye, A. S. Mahmood, C. Awortwe and A. K. Nyarko, Interdiscip. Toxicol., 2015, 8, 193–202 CrossRef CAS PubMed.
- Y. Yuan and H. Chen, Food Packag. Shelf Life, 2021, 30, 100718 CrossRef CAS.
- M. Rosin, A. Welk, O. Bernhardt, M. Ruhnau, F. A. Pitten, T. Kocher and A. Kramer, J. Clin. Periodontol., 2001, 28, 1121–1126 CrossRef CAS.
- Z. Zhou, D. Wei, Y. Guan, A. Zheng and J. J. Zhong, Mater. Sci. Eng., C, 2011, 31, 1836–1843 CrossRef CAS.
- J. Guo, J. Qin, Y. Ren, B. Wang, H. Cui, Y. Ding, H. Mao and F. Yan, Polym. Chem., 2018, 9, 4611–4616 RSC.
- M. S. Ganewatta and C. Tang, Polymer, 2015, 63, A1–A29 CrossRef CAS.
- A. A. Overview, Y. Pan, Q. Xia and H. Xiao, Polymers, 2019, 11, 1238 CrossRef PubMed.
- R. Namivandi-Zangeneh, R. J. Kwan, T.-K. Nguyen, J. Yeow, F. L. Byrne, S. H. Oehlers, E. H. Wong and C. Boyer, Polym. Chem., 2018, 9, 1735–1744 RSC.
- M. R. E. Santos, P. V. Mendonça, M. C. Almeida, R. Branco, A. C. Serra, P. V. Morais and J. F. J. Coelho, Biomacromolecules, 2019, 20, 1146–1156 CrossRef CAS PubMed.
- S. Barman, M. M. Konai, S. Samaddar and J. Haldar, Appl. Mater., 2019, 11, 33559–33572 CrossRef CAS.
- S. K. Sharma and G. S. Chauhan, J. Mater. Sci.: Mater. Med., 2010, 717–724 CrossRef CAS PubMed.
- G. Garg, G. S. Chauhan, R. Gupta and J. Ahn, J. Colloid Interface Sci., 2010, 344, 90–96 CrossRef CAS.
- P. Copolymer, Y. Xue and H. Xiao, Polymers, 2015, 7, 2290–2303 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |