Open Access Article
Open Access ArticleContinuous flow mechanochemistry: reactive extrusion as an enabling technology in organic synthesis
Robert R. A.
Bolt
 a,
Jamie A.
Leitch
a,
Jamie A.
Leitch
 *a,
Andrew C.
Jones
*a,
Andrew C.
Jones
 b,
William I.
Nicholson
b and
Duncan L.
Browne
b,
William I.
Nicholson
b and
Duncan L.
Browne
 *a
*a
aDepartment of Pharmaceutical and Biological Chemistry, UCL, School of Pharmacy, 29-39 Brunswick Square, Bloomsbury, London, WC1N 1AX, UK. E-mail: j.leitch@ucl.ac.uk; Duncan.browne@ucl.ac.uk
bCardiff Catalysis Institute, School of Chemistry, Cardiff University, Main Building, Park Place, Cardiff, CF10 3AT, UK
First published on 4th May 2022
Abstract
Rapid and wide-ranging developments have established mechanochemistry as a powerful avenue in sustainable organic synthesis. This is primarily due to unique opportunities which have been offered in solvent-free – or highly solvent-minimised – reaction systems. Nevertheless, despite elegant advances in ball-milling technology, limitations in scale-up still remain. This tutorial review covers the first reports into the translation from “batch-mode” ball-milling to “flow-mode” reactive extrusion, using twin-screw extrusion.
Key learning points(1) There can be limitations to the scale of ball milling mechanochemistry.(2) Reactive extrusion, most commonly twin-screw extrusion (TSE), can provide a scalable continuous solution. (3) TSE allows for the fine tuning of reactor design and assessment of other continuous variables. (4) TSE in organic synthesis is still in its infancy. (5) Further development in TSE will lead to increased adoption of continuous mechanochemistry in industry. |
1. Introduction
The development of continuous methods for the processing of chemicals has revolutionised large-scale synthesis, especially in industrial settings.1 Furthermore, advances in the translation from “batch” to continuous “flow” mode have expanded rapidly, offering valuable opportunities for a variety of research areas including the handling and scaling of hazardous reactions,2 development of flow photochemistry manifolds,3 and the safe introduction of gases4 (Scheme 1A); the benefits of continuous processing have been reviewed extensively in the literature.5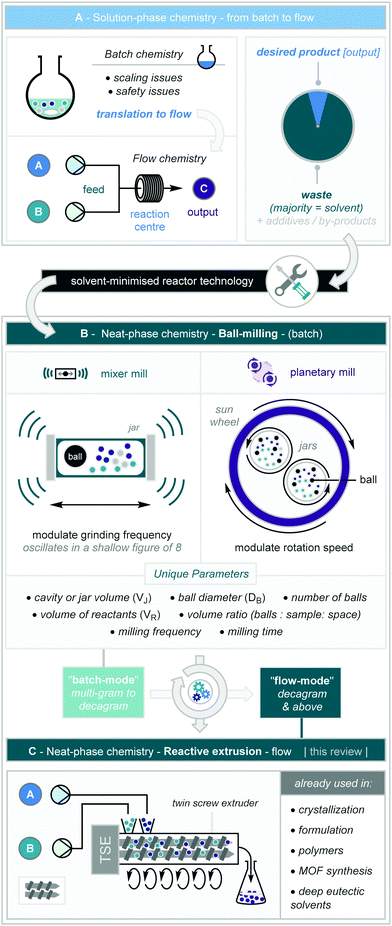 | ||
| Scheme 1 Reactive extrusion in the context of batch and continuous solution-phase chemistry and solid-state ball milling. | ||
Whilst these advances have continued to transform contemporary synthesis, they suffer from a key aspect of green chemistry in that bulk solvent is still required to enable effective reactivity.6 Furthermore, low reaction concentrations are routine within flow chemistry to allow effective solubilisation of reaction components and prevent precipitation, clogging or fouling of the flow tubes.7 The dogmatic use of solvent – and often undesired toxic solvents such as DCM and DMF – has established a status quo where synthetic chemists are comfortable with the fact that the majority of their molecular input to a reaction flask (solvent), eventually leads – directly or indirectly – to atomic waste.8 The growing importance of sustainability metrics such as atom economy, e-factor, process mass intensity, and space time yields in industrial route design and development,9 has allowed researchers to take a detailed look at the “desired output”/“waste” dichotomy, as the use of bulk solvent plays an important role in the latter two of these metrics. Accordingly, reducing the quantities of solvents, and the opportunity of removing them completely from the reaction medium – to conduct chemical reactions “neat” or in the solid-state – is incredibly pertinent from the perspective of sustainable chemistry.10
Within this context, the ability to enact material change on chemicals through application of mechanical force has been known for millennia through utility of grinding tools such as pestles and mortars.11 More recently, such application of mechanical force on chemicals – known as mechanochemistry – has become well-established within the areas of crystal engineering and formulation science. This has stimulated the adoption of mechanochemical equipment such as mixer mills and planetary mills (Scheme 1B). Using balls (made from a variety of materials) in jars (also made from a variety of materials), agitation of these jars via oscillation/rotation causes the balls to move within the jar imparting compressing mechanical forces on the contents. These are primarily impact forces in mixer mills and shearing forces in planetary mills. Further to this, the implementation of mechanochemical techniques introduces unique reaction parameters including: cavity volume (VJ), ball diameter (DB, which influences mass), number of balls, volume of reactants (VR), volume ratios and milling frequency.
Over the last few decades, synthetic chemists have turned to existing mechanochemical equipment and technology to enable solvent-free – or highly solvent-minimised – synthesis.12 For these reasons, mechanochemical techniques have been coveted for its applications in sustainable synthesis and has been highlighted for its adherence to the 12 principles of green chemistry.13
Moreover, these protocols have been demonstrated to offer favourable reactivity design profiles through drastically reduced reaction times, the possibilities of carrying out often-sensitive reactions under an air atmosphere,14 and improved/complementary selectivity profiles.15 Despite this, the use of high-speed ball mills to carry out these transformations have an upper limit in reaction scale from a safety perspective. Whilst larger mills are available for crushing rocks and extracting minerals, scaling synthetic organic reactions in the fine-chemicals sector comes with a requirement for controlling exo- and endo-therms. Reactions at these scales may also lead to typical batch scale up issues of localised hot spots, large inventory of potential hazard and high demand on temperature control. Indeed, many of the latter batch considerations can be amplified in the absence of a solvent, solvents often act to improve mixing and enable more effective thermal dissipation. For these reasons, in order to explore the full opportunity of mechanochemical synthesis, and to increase the potential for industrial adoption, translation from “batch-mode” ball milling to “flow-mode” reactive extrusion is paramount (Scheme 1C).
Extrusion equipment has been routinely employed for decades in both the food (notably for the preparation of dried pasta and breads) and the polymer industries. Furthermore, the manipulation of solids and/or liquid chemicals via reactive extrusion has already been widely studied in crystallization (especially co-crystallisation), final-form preparation, MOF and supramolecular porous cage synthesis, and also in the preparation of deep eutectic solvents.16 To this end, in 2019 – alongside flow chemistry – reactive extrusion was highlighted by IUPAC as one of the 10 most important chemical innovations that could change our world.17 Crossing the interface of organic chemistry and engineering science, a significant barrier to adoption for organic synthesis applications has been witnessed. To this end, this review will aim to break down these barriers, covering the first forays into the design and development of reactive extrusion methodology within the context of molecular organic synthesis. Following this, based on our findings and our own studies, we have formulated a “how-to” protocol for designing extrusion reactions.
2. Reactive extrusion
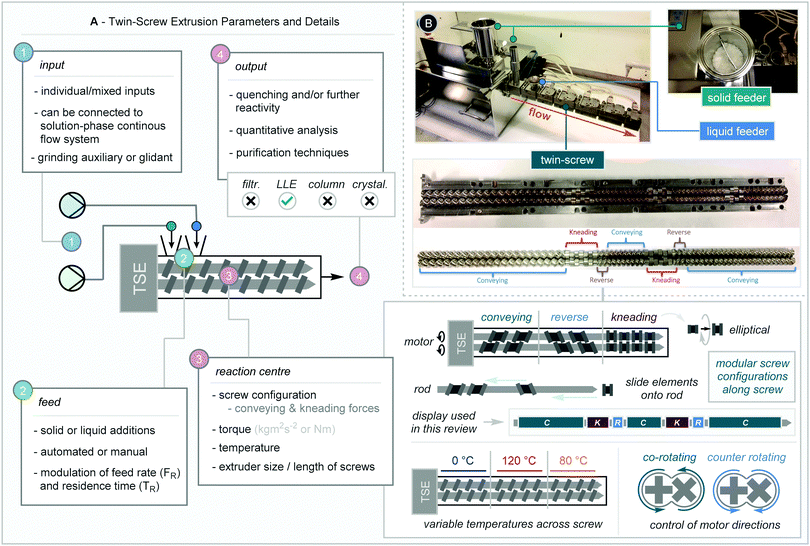
The most common type of reactive extrusion uses a twin-screw extruder. This screw-based system can be divided into four distinct areas to consider; input, feed, reaction centre, and output (Scheme 2A). Each area offers its own distinct variables and parameters that must be considered. Herein we present the pioneering work in this field and bring together observations and possible learnings that appear to be collectively demonstrated in the context of extrusion. It should be noted that screw-based “batch” mixers have been used previously in the synthesis of OLED materials,18 however the continuous extruder will be the focus of this tutorial review.2.1. Input
Prior to addition to the extruder, compounds can be slurried together, mixed with a pestle and mortar, and even mixed in a ball mill to evenly distribute material. Furthermore, solution-phase continuous flow systems can be linked to allow addition of reagents through liquid feeders (Scheme 2B). Addition of solvents as liquid-assisted grinding (LAG) agents can assist in screw flow, in turn stopping blockages. Furthermore, solid additives known as glidants (similar to grinding auxiliaries in smaller scale batch mechanochemistry) can be added to facilitate flow and/or serve as a reaction surface.2.2. Feed
Following this, materials can be added to twin-screw extruders via feeders located either before or during the screw set-up. These are normally either solid (where you can also add slurries) or liquid feeders (where compounds are added via syringe or HPLC pump). The additions can be both manual or automated (via a syringe pump for example), and the latter affords the user control over the feed rate (FR) which has a direct influence on the residence time (TR) in the screw.2.3. Reaction centre
The reaction centre of the twin-screw extruder offers a plethora of variables to consider. The screws are rotated by a motor which can be used to modulate screw speed (which will affect TR) and the torque of the screw (which will also be dependent on the nature of the reactants). Users should avoid allowing the screws to be “torqued out” which shuts down rotation due to paste formation, gumming, or blockages, in turn nullifying reactivity (the latter can also be achieved by feeding material too quickly into the reaction centre). The screws themselves can be configured with “conveying”, “reverse” and “kneading” elements. These elements can be configured in a “plug-and-play” system and are simply slid along the screw rod to configure. Moreover, kneading elements can be positioned at 30, 60 (in either a forward or reverse orientation), and 90 degrees, and it is widely understood that these elements impart the greatest mechanical forces onto materials. After they have been configured, in certain extruders these screws can then be rotated either in a “co-rotating” or a “counter-rotating” fashion which will also exert different mechanical forces. Importantly, different sections along the length of the extruder can be heated to different temperatures. Whilst predominately a single temperature can be utilised, this facet enables fine-tuning of reaction optimization, especially in cascade reactions (vide infra).2.4. Output
The mixture that is ejected from the screw is often a powder/paste and can be further manipulated via quenching, further reactivity, or undergo purification. At this point, analysis of the crude reaction mixture, including both standard solution phase methods such as NMR spectroscopy, but also powder X-ray diffraction (PXRD), can take place. In this review, how the reaction mixture is purified will be highlighted, whether it is filtration (filtr.), liquid–liquid extraction (LLE), column chromatography, or through a recrystallisation/crystallisation protocol (crystal.)Other variations on the extruder including home-made devices have been employed and further details will be given where appropriate.
The key papers to date will now be discussed in a case-study style format and details of sustainability/reactor metrics such as space time yield will be given where available. Furthermore, comparisons to solution phase and “batch-mode” ball milling will also be given where available.
3. Reactive extrusion in synthesis
3.1. Crawford & James – 2017
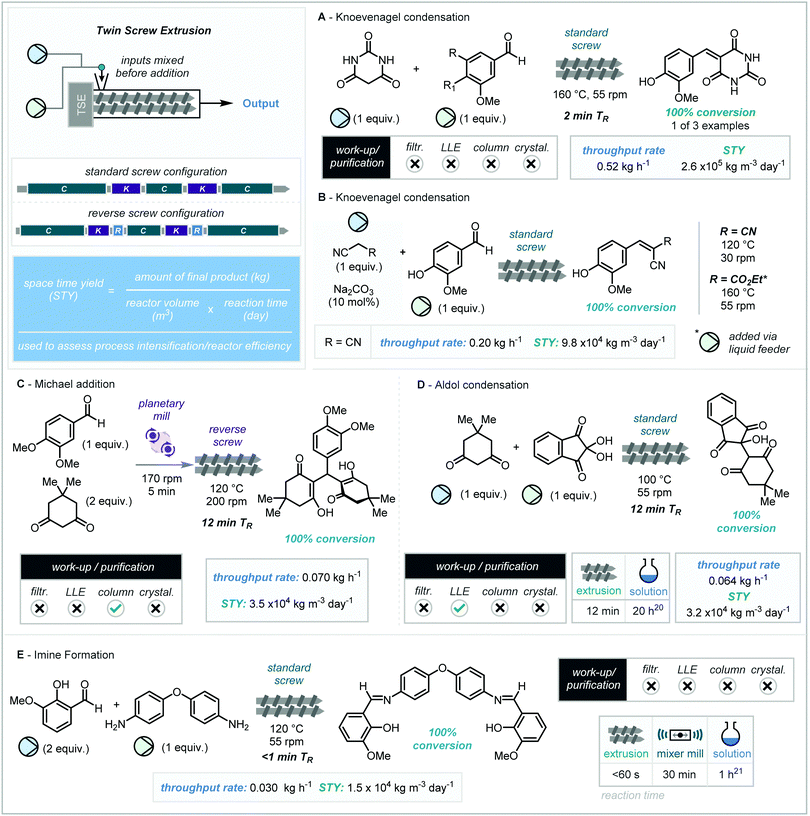
Despite the utility of extrusion equipment in other fields of chemistry, remarkably it wasn’t until 2017 when the first studies in molecular organic synthesis were reported. The Knoevenagel reaction between vanillin and barbituric acid has become an archetypal study for ball-milling reactions. Crawford, James, and co-workers reported a translation of a Knoevenagel reaction for scale up via twin-screw extrusion.19 Initial investigations using a screw system made-up of conveying and kneading sections (standard screw) showed that – after pre-mixing the starting materials and feeding them into the extruder – the reaction could successfully be achieved at 160 °C with a screw speed of 55 rpm.Interestingly, no reaction was found to occur at low temperature <40 °C. This is in part attributed to the melting point of the staring materials. Differential scanning calorimetry (DSC) and contact angle experiments uncovered that the wetting of barbituric acid by the aldehyde (vanillin) is necessary for effective reactivity. Therefore, increased temperatures of that above vanillin's melting point (>83 °C), allowing for lubrication of the reaction mixture, was shown to be optimal and this system was applied to a small range of vanillin derivatives exhibiting a range of melting points (40–166 °C). Notably, the only by-product in the reaction mixture was water, which was removed as steam within the screw barrel.
The authors calculated space time yields (STY, kg m−3 day−1) for each extrusion reaction (equation located at Scheme 3, top left), a metric which can be utilized in examining reactor efficiency and process intensification of a reaction. Larger STY values denote improved efficiency; and favorable STY metrics can drive industrial route design. From a throughput rate of 0.52 kg h−1 (from a feed rate of 0.60 kg h−1), the phenol example shown (Scheme 3A, top) was found to have an impressive space time yield of 2.6 × 105 kg m−3 day−1. For comparison, a representative solution phase reaction, processing 1 g of a 500 g mol−1 material in 20 mL (0.25 M) solvent in a 50 mL flask with a 12 hour reaction time would give a STY of 40 kg m−3 day−1 (480 kg m−3 day−1 if full conversion observed in 1 hour).
 | ||
| Scheme 3 Twin-screw extrusion as an enabling technology in solid state organic synthesis.20,21 | ||
Following this successful translation from “batch-mode” ball-milling to “flow-mode” extrusion, the reaction of vanillin and malononitrile (mp = 32 °C) was explored. Subjecting this reaction to the above optimized conditions (55 rpm, 160 °C) resulted in no product formation, attributed to unproductive polymerization of malononitrile. To circumvent this issue, the authors introduced Na2CO3 (10 mol%) as a catalyst which permitted the use of a lower temperature of 120 °C, which in turn enabled complete conversion to the condensation adduct. It must be noted the output of this reaction was not separated from the carbonate catalyst. Furthermore, the reaction of liquid inputs was also shown to be compatible with this process with the reaction of vanillin with ethyl cyanoacetate. In this case the vanillin and sodium carbonate were introduced via the solid feeder and the cyanoacetate was injected into the liquid feeder via syringe pump (Scheme 3B). Reaction at 160 °C at a screw-speed of 55 rpm gave complete conversion to product allowing for a space time yield of 5.6 × 104 kg m−3 day−1.
Even with preliminary optimization studies into the ball-milling enabled cascade condensation/Michael addition between vanillin and dimedone, conversions of only 7.5% were obtained. Despite this, as TSE can introduce the ability to fine-tune temperature control and screw configuration, the authors elected to explore this methodology further in a continuous setting. All screw speeds (30–250 rpm) at room temperature resulted in no conversion. An elevated temperature of 40 °C at 55 rpm resulted in a visual and physical change, producing a golden yellow liquid and a conversion of <5%. After further increase of temperature, the addition of reverse segments to the screw configuration after kneading segments – a technique to increase residence time and kneading duration – was found to be key, obtaining up to a 98% conversion at 120 °C and 200 rpm (Scheme 3C). In addition, complete conversion was only achieved when the reagents were premixed in a planetary mill (170 rpm, 5 minutes). This new reverse screw configuration resulted in an extended residence time (12 minutes) leading to a throughput rate of 0.070 kg h−1 with a STY of 3.5 × 104 kg m−3 day−1. However, due to the formation of unidentified by-products, purification via flash column chromatography was required.
Kaupp and co-workers have previously shown the aldol reaction of ninhydrin and dimedone to provide quantitative product without the need for further purification.22 This reaction was then investigated by James, Crawford and co-workers by TSE (Scheme 3D). It was found once again, that Na2CO3 (10 mol%) was required. Complete conversion to product could be achieved at 100 °C, however a low feed rate (0.8 g min−1) was required to overcome an undesired increase in torque. Both ball milling and TSE methodologies have been shown to be superior to the analogous solution reaction which proceeds to only 78% yield overnight at reflux, highlighting that TSE provides an excellent opportunity for continuous synthesis.
Finally, the synthesis of C–N bonds through condensation to form an imine has previously been demonstrated mechanochemically in batch mode. For TSE application, screw speeds between 55 and 250 rpm were performed at room temperature, with all speeds above 55 rpm resulting in decreased conversion (<28%) due to decreasing residence times. Alteration of barrel temperature resulted in complete conversion at 80 °C. Having previously been added manually the reagents were now introduced automatically at set rates. Below 0.45 g min−1 gave no conversion with subsequent rates of 0.73 g min−1 and 0.79 g min−1 giving conversions of 70% and 90% respectively. Complete conversion was achieved using a temperature of 120 °C (Scheme 3E). No purification was required, resulting in analytically pure product produced at a throughput rate of 0.030 kg h−1 and an STY of 1.5 × 104 kg m−3 day−1. This report constituted an important step in showcasing what can be achieved using extrusion equipment in molecular organic synthesis.
3.2. Isoni – 2017
Whilst continuous reduction processes utilizing HPLC pumps and pre-packed columns (packed with Celite, NaBH4 and LiCl) have been established by Seeberger and co-workers, this system was limited to gram-scale, and also required organic solvents and regular regeneration of packed columns.23 To this end, Isoni and co-workers proposed and developed a “greener” alternative for aldehyde reduction, capable of multikilogram continuous scale operation.24Initial optimization was conducted using water insoluble aldehydes (such as 4-chlorobenzaldehyde) and an improvised high-shear reactor (550 W, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, food blender) on gram-scale (Scheme 4A). It was found that the reaction could be performed in the absence of organic solvents and in less than one minute with variable conversion (25–95%), and upon addition of aqueous 1 M NaOH conversion increased further (>98%). Using the model reaction conditions from above (summarized in Scheme 4B), the reaction was then performed on pilot kilogram scale (Scheme 4C) passing the slurried mixture under a nitrogen atmosphere through a modified twin-screw extruder, producing 1.41 kg of the benzyl alcohol product in just 17 minutes (4.53 kg h−1) with high purity (89%), where simple vacuum filtration and washing with water was all that was required to obtain the reduced alcohol. Inspection of the equipment at the end of the continuous run highlighted fouling of the NaBH4 slurry inlet nozzle which led to a decrease in conversion over the duration of the run. Moreover, it was noted that a temperature increase of 15 °C (23 to 38 °C) in less than 5 minutes of operation was observed and thus further reactor design and engineering would be required to more reliably control this scale up process. The authors did however detail several key potential benefits over batch alternatives, which include: (a) 22 times greater volume efficiency; (b) 11 times volume efficiency (including work-up); (c) more than 33 times more energy efficiency (MJ per ton product); (d) no organic solvent used (THF in batch) however more NaOH use (potential to recycle this in TSE); (e) OPEX (Operating expenses) were more than 16 times greater for batch.
000 rpm, food blender) on gram-scale (Scheme 4A). It was found that the reaction could be performed in the absence of organic solvents and in less than one minute with variable conversion (25–95%), and upon addition of aqueous 1 M NaOH conversion increased further (>98%). Using the model reaction conditions from above (summarized in Scheme 4B), the reaction was then performed on pilot kilogram scale (Scheme 4C) passing the slurried mixture under a nitrogen atmosphere through a modified twin-screw extruder, producing 1.41 kg of the benzyl alcohol product in just 17 minutes (4.53 kg h−1) with high purity (89%), where simple vacuum filtration and washing with water was all that was required to obtain the reduced alcohol. Inspection of the equipment at the end of the continuous run highlighted fouling of the NaBH4 slurry inlet nozzle which led to a decrease in conversion over the duration of the run. Moreover, it was noted that a temperature increase of 15 °C (23 to 38 °C) in less than 5 minutes of operation was observed and thus further reactor design and engineering would be required to more reliably control this scale up process. The authors did however detail several key potential benefits over batch alternatives, which include: (a) 22 times greater volume efficiency; (b) 11 times volume efficiency (including work-up); (c) more than 33 times more energy efficiency (MJ per ton product); (d) no organic solvent used (THF in batch) however more NaOH use (potential to recycle this in TSE); (e) OPEX (Operating expenses) were more than 16 times greater for batch.
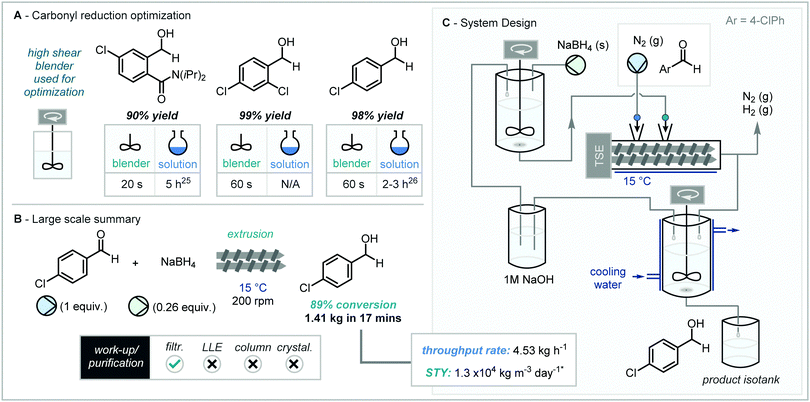 | ||
| Scheme 4 Twin-screw extrusion in the aqueous reduction of aldehydes, home-made high shear blender used instead of a ball mill for assessment of reaction in batch mode.25,26 * Space-time yield metric calculated using parameters given by Isoni and co-workers. | ||
3.3. Crawford & James – Nov 2017
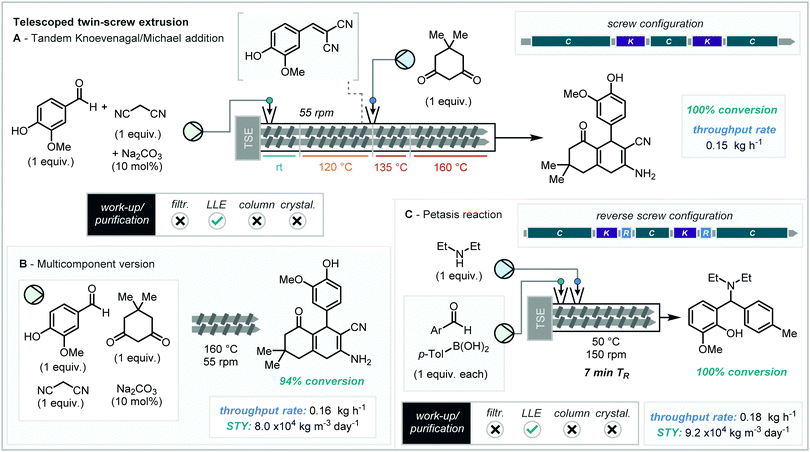
The ability to carry out multi-step reactions in continuous extrusion has already been exemplified by Crawford, James and co-workers, and in a subsequent report, the authors expanded this concept to demonstrate how multiple subsequential reactions can be telescoped together with one device through the addition of successive reagents further along the extrusion barrel (Scheme 5A).27 This was elegantly showcased using the Knoevenagel condensation of vanillin and malononitrile followed by the subsequent Michael addition upon the introduction of dimedone.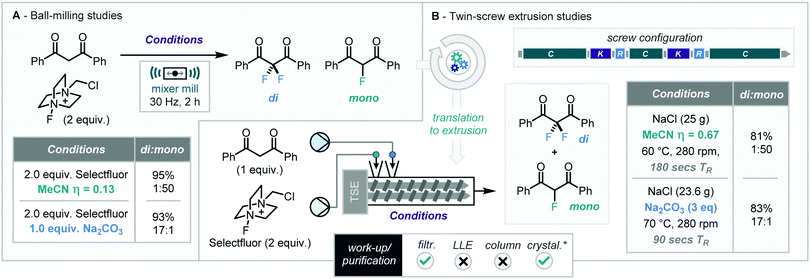 | ||
| Scheme 6 Twin-screw extrusion enabled fluorination of 1,3-dicarbonyls. * recrystallisation only used on di-fluorinated species. | ||
Using the previously optimized Knoevenagel condensation conditions (for details, see Section 3.1)19 and sub-stoichiometric quantities of Na2CO3, 120 °C and a screw speed of 55 rpm afforded complete conversion. When exploring the Michael addition step, alteration of the reaction temperature was important and was studied at intervals between 25 and 160 °C. Notably, for a telescoped process in a single extruder, because the screw speed is set for the process as a whole rather than individual sections of the screw/barrel, temperature control becomes the key variable. Interestingly, below 140 °C the reaction was shown to be ineffective, however above this the product formed with maximum conversion at 160 °C owing to the formation of a melt. However, some notable issues that arose were (1) the formation of “bridges” at the feeders due to temperatures being greater than that of the melting point of the input feeds and (2) abnormally long (40 minutes) residence time due to uncontrollable fluctuation in reaction rheology. These were resolved by the temperatures at the feeder locations being lowered below that of the reagents melting points and the use of lower feed rates. This led to uncovering a “one-screw” cascade reaction affording the addition adduct in 100% conversion. This system also serves to showcase one of the unique practicible points of extrusion technology, in that fine-tuning of the temperature of each individual section is possible.
Following this, a multi-component equivalent analogous method was sought, by mixing all three reaction components together with the base catalyst, using a screw speed of 55 rpm and a temperature of 160 °C, the same adduct was produced in excellent conversion (Scheme 5B). These multi-component reaction (MCR) conditions also resulted in a slightly greater throughput rate of 0.16 kg h−1 and a STY of 8.0 × 104 kg m−3 day−1.
Further to these findings, Crawford, James and co-workers also demonstrated the Petasis reaction by twin-screw extrusion, which notably had not been mechanochemically performed in batch before. Initial use of the standard screw configuration resulted in low conversions to product (<20%). Substitution to a reverse screw configuration amplified the duration of both kneading segments, leading to increased reaction efficiency. Optimisation of the screw temperature and speed resulted in the crucial conditions of 50 °C and 150 rpm to ensure complete conversion (Scheme 5C). These conditions produce a residence time of just 7 minutes, equivalent to a throughput rate of 0.18 kg h−1 and a STY of 9.2 × 104 kg m−3 day−1. The boron-based by-product was then readily removed via a liquid–liquid extraction using ethyl acetate and water to generate the pure product.
3.4. James & Browne – 2018
Fluorinated compounds – and methods for their efficient synthesis – have become ever more important for many chemical sectors due to the numerous documented positive impacts that the inclusion of fluorine atoms can have on many functional molecules, such as improving the efficacy of pharmaceutical and agrochemical compounds.28 These structures are generally accessed via either electrophilic or nucleophilic fluorination methodologies using reagents such as Selectfluor or NFSI (as substitutes to F2) or various fluoride sources. Many fluorinated compounds can be attained through building blocks such as fluorinated 1,3-dicarbonyls but production of these compounds can suffer with selectivity issues due to potential mono and di-fluorination at the acidic 2-position, which remains a substantial challenge necessitating careful control of reaction conditions and system design. Browne and co-workers have developed a range of highly selective protocols for the synthesis of both fluorinated building blocks under ball milling conditions including, heterocycles and 1,3-dicarbonyls.29 In 2016, while investigating the fluorination of 1,3-diketones they found excellent reaction selectivity for producing either the mono or difluorinated products using additive control in solvent-free/minimised mechanochemistry. Here addition of MeCN (η = 0.13 mL g−1 of total mass of reagents) as a liquid additive selectively produces the mono-fluorinated species whereas use of Na2CO3 (1.0 equiv.) afforded selective formation of the di-fluorinated species.Successful translation to twin-screw extrusion from the mixer mill using the same additive-dependent switch was achieved maintaining the excellent selectivity observed previously (Scheme 11).30 Key to the adaption of this reaction was the increased reaction temperature (60–70 °C) along with the introduction of NaCl as a glidant (grinding agent) (Scheme 6).
 | ||
| Scheme 7 Bulk solvent and CRM-free peptide coupling enabled by recirculating screw extrusion. aSolution values calculated using NEt3 as base and DMF as solvent. | ||
 | ||
| Scheme 8 Use of single screw extruders in organic synthesis.36,37 | ||
 | ||
| Scheme 10 Synthesis of naphthalene diimide (NDI) pigments via twin-screw extrusion. a Batch STY calculated assuming reaction space is twice volume of solvent used. | ||
Using a screw configuration including conveying, kneading, and reverse sections, continuous extrusion was demonstrated to produce difluorinated products, after filtration and recrystallisation from cyclohexane, in 70% isolated yield (values given in Scheme 6 are NMR yields) at a rate of 3.94 kg h−1. This is a stark increase when compared to 0.14 kg h−1 that could theoretically be achieved under the optimized ball-milling conditions. Similarly, the mono-fluorinated diketone was formed in an excellent yield up to 90%, corresponding to a high throughput rate of 5.14 kg h−1, isolated as a mixture of isomers through simple filtration. This work demonstrates the potentially facile nature of process intensification which can be achieved through the application of the extruder technology to previously established ball milling procedures. Furthermore, it shows the potential for the use of this solvent free continuous method for the construction of fluorinated building blocks.
3.5. Métro – 2018
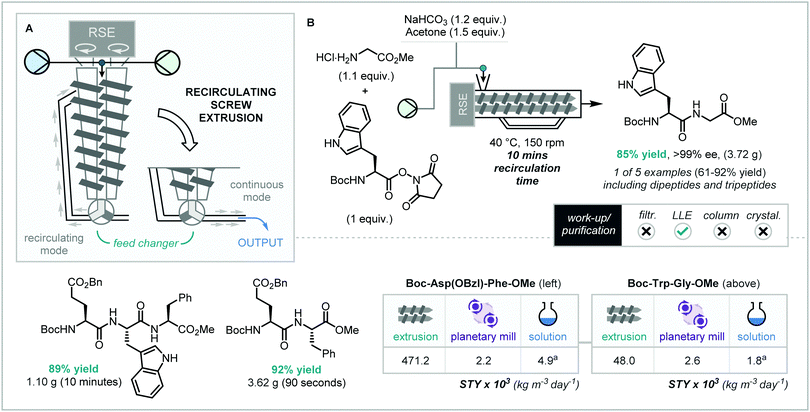
The importance of the development of efficient, atom economical, and sustainable methods for the forging of peptide bonds in industrial settings cannot be overstated. Two of the major research avenues that have been employed to provide solutions to the above are (a) methods negating the need for stoichiometric activating reagents, which in turn produce large quantities of atomic waste, (b) the development of solvent-free amide coupling reaction methodology, through mechanochemical techniques such as ball-milling. To this end, in 2009, Lamaty and co-workers described the solvent-free synthesis of key peptide bonds, via the ball-milling-enabled coupling of α-amino ester salts with urethane-protected α-amino acid carboxyanhydride (UNCA) using simple NaHCO3 as base.31 Following this, a selection of further ball milling processes have been described in the literature for the formation of amide bonds, most often employing amide coupling agents such as EDC and CDI. Within this context, in 2018, Hernandez and co-workers described the papain-catalyzed homo-oligomerization of amino acid residues using twin-screw technology.32 Notwithstanding this exception, all of the mechanochemical amide bond formation reactions have been hampered by scale through reliance on “batch-mode” ball milling.In 2018, Métro and co-workers reported an important step towards the continuous solvent-free synthesis of di- and tri-peptides via reactive extrusion.33 Furthermore, the authors targeted a CMR-free (carcinogenic, mutagenic, or reprotoxic) method – requiring the omission of not only toxic coupling reagents but also ubiquitous solvents such as DMF, DCM, and THF. For the extrusion process a co-rotating twin-screw extruder (MC15 microcompounder, Xplore) containing a feed recycler – dubbed “recirculating screw extrusion” in this review – was employed (Scheme 7A). Whilst mimicking twin-screw extruders discussed above, here, a recirculating loop can be brought into play, allowing recirculation of the reaction mixture which leads to extended reaction/residence times. After the desired recirculation time, the feed changer can be switched to eject the reaction mixture. The authors optimized on a model system of the peptide coupling of the activated ester Boc–Trp–OSuc and HCl·H–Gly–OMe, and stemming from preliminary planetary mill results, the authors demonstrated that the addition of acetone (η = 0.15 mL g−1) as a liquid assisted grinding (LAG) agent was crucial for efficient flow through the extrusion chamber. Furthermore, using a recirculating time of 10 minutes, heating the chamber to 40 °C, and a screw speed of 150 rpm, 85% yield of Boc–Trp–Gly–OMe (3.72 g) was achieved with 99% ee at the stereogenic center maintained. As means of post-extrusion manipulation (which was also used in other examples), the dense paste ejected from the extruder, was dried overnight under reduced pressure over P2O5, then submitted to liquid–liquid extraction work-up, which – after concentration in vacuo – in all cases afforded pure peptide product without the need for further purification. This methodology was expanded to the coupling of HCl·H-Phe-OMe with Boc-Trp-OSuc and Boc-Asp(OBzl)-OSuc to form the dipeptide structures in 61% and 92% yield respectively.
By simple submission of the dipeptide products formed above to an atmosphere of dry HCl overnight to remove the Boc protecting group, the free amine dipeptides could then be resubmitted to the extrusion methodology forming tripeptides Boc-Asp(OBzl)-Trp-Gly-OMe and Boc-Asp(OBzl)-Trp-Phe-OMe in excellent yield (>86%), and purity (>94%).
When the space-time yield values for the RSE-enabled peptide coupling were compared to “batch-mode” planetary ball mill and solution-phase counterparts, remarkable 20–200 fold increases were observed. Even more notable is that the authors benchmarked their extrusion work against solution phase comparisons which employed industrially undesired NEt3 and DMF reaction conditions. When compared to solution phase reactions using NaHCO3 as base and acetone as solvent, a 2356 fold increase was observed. These STY results – alongside the sequential coupling reactions, and the application of this methodology to the synthesis of peptide fragments such as aspartame – showcase the potential opportunities for industrial production of peptide sequences using reactive extrusion.
3.6. Kulkarni – 2019
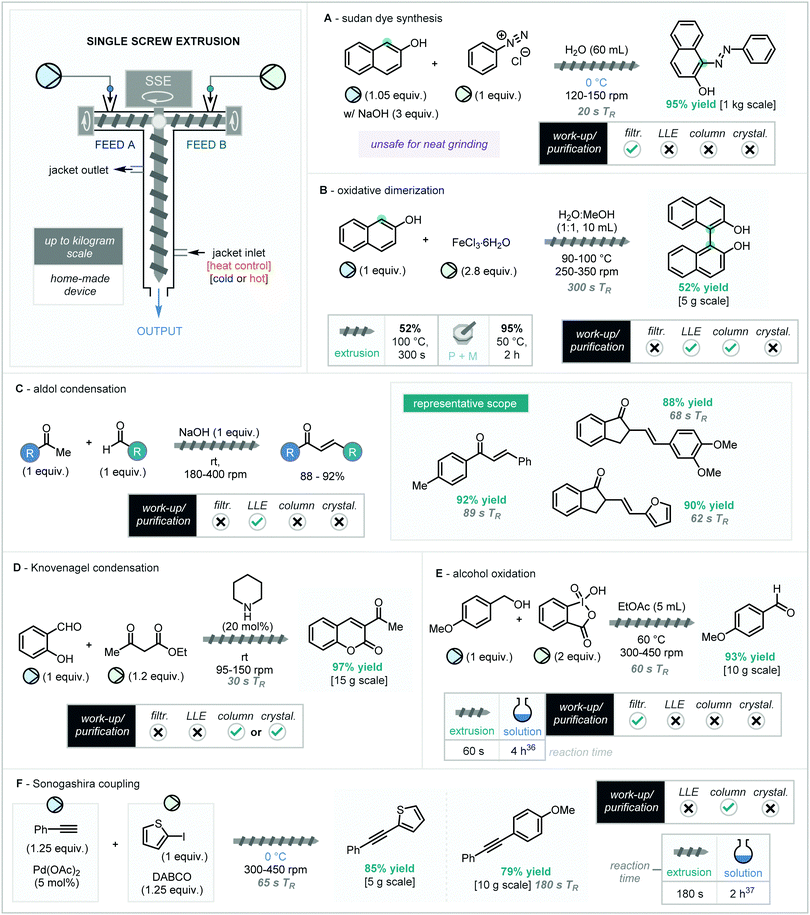
As an alternative to the use of two screws to impart continuous extrusion, Kulkarni and co-workers designed and built a distinct jacketed single vertical screw reactor (Scheme 8), particularly useful for neat solids with solubility issues as well as slurries.34 The unique design, based on a Teflon-screw inside a condenser-type glass container, allows for precise temperature control of the extrusion process as well as the ability to monitor the visual changes over the reaction due to a see-through reactor wall, both of which lead to increased safety and efficiency compared to previous methodology. Furthermore, the vertical orientation is preferred to remove the potential for unsafe generation of friction. The introduction of the reagents is by the direct addition to the feeder screws or via a pump for fluid reagents. Reactions can also be performed under solvent-free or solvent-minimised conditions and this system is designed to process solid mixes, pastes and slurries alike. The authors applied this novel single-screw extruder to a portfolio of nine distinct organic transformations, including catalytic reactions and redox adjustments. Notably this screw system (like the RSE above) do not contain kneading screw sections, suggesting mechanical force is elicited through shearing against the wall of the reactor.The synthesis of azo dyes and their associated diazonium intermediates has previously been explored in continuous flow, pestle and mortar grinding as well as solid support reactivity. However, these techniques generally either require polar solvents (including DMF) or are unsafe for large scale production of dry diazonium salts due to well-established sensitivity towards friction, heat or shock. To this end, the production of the popular azo dye, Sudan dye has been achieved under continuous solvent-minimised conditions using Kulkarni's screw design (Scheme 8A). Firstly, the diazo reagents were synthesised in a tubular reactor before being fed directly into the single screw extruder (SSE) along with 2-naphthol. It was found that the addition of NaOH and water was crucial due to the formation of a basic media. The reaction was maintained at 0 °C with a screw speed between 120–150 rpm. Complete conversion was observed after only 20 s as a red slurry, which required further filtration to afford the desired dye in 95% yield on a 25 g scale. Remarkably, the scalability of this process was highlighted through the synthesis of nearly 1 kg of the target dye along with the potential to scale-up further to 500 kg should the equipment run continuously for 7 days.
BINOL and its derivatives provide the backbone to many privileged ligand systems. Synthesis is often achieved via oxidative homocoupling of 2-naphthol in the presence of an oxidant such as FeCl3·6H2O. Batch reaction has previously been achieved by grinding using a pestle and mortar followed by heating at 50 °C for 2 hours.35
Application to SSE at 50 °C gave no product formation but raising this temperature to 160 °C at a screw speed between 830–900 rpm pleasingly afforded the crude product in 50 s leading to 38% of the pure product after purification (silica gel flash column chromatography). It was noted that the poor yield is due to the formation of a semisolid/adhesive solid at elevated temperatures, which consequently gives poor mixing/heat transfer. The addition of a solvent (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 1
MeOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) along with the use of lower temperatures (90–100 °C) and a lower screw speed of 250–350 rpm reduced these effects allowing for the formation of a more free flowing paste and thus more efficient mixing. This gave an increased yield of 52%, although coupled with an increase in residence time of 300 s (Scheme 8B).
1) along with the use of lower temperatures (90–100 °C) and a lower screw speed of 250–350 rpm reduced these effects allowing for the formation of a more free flowing paste and thus more efficient mixing. This gave an increased yield of 52%, although coupled with an increase in residence time of 300 s (Scheme 8B).
The aldol condensation remains an integral reaction in organic synthesis and has already been explored on multiple occasions in extrusion technology (Section 3.1) including the synthesis of chalcones which has been performed using multiple techniques. Using SSE, the solvent minimised synthesis was achieved using 4-methyl benzaldehyde and acetophenone along with one equivalent of NaOH at 180–200 rpm (Scheme 8C). The crude product was afforded with a residence time of 89 s, with the desired chalcone obtained in 92% yield after washing with water. It was noted the starting materials are liquid and the product solid, allowing for a crude visualisation of reaction progression. The application of this continuous chalcone synthesis could be extended to reaction with veratraldehyde (88%) and furfural (90%) using residence times of 68 s and 62 s respectively.
Related to the aldol condensation is the Knoevenagel condensation, which has previously been explored in continuous extrusion (TSE) by James and co-workers for the reaction of aldehydes with barbituric acid (Scheme 3). The continuous synthesis of a valuable coumarin (previously achieved in batch by mortar and pestle)38 has been demonstrated by Kulkarni using the SSE (Scheme 8D). The reaction of salicylaldehyde, acetyl acetone and piperidine gave the desired 3-acetyl coumarin in excellent yield (97% after column) after a residence time of 30 s at 95–150 rpm. The reaction of all liquid starting materials started to solidify after just 6 s indicating rapid initiation of the reaction.
The SSE has also been applied to the oxidation of primary alcohols (Scheme 8E). Upon addition of 4-methoxy benzyl alcohol and IBX (2 equivalents), the reaction initiated almost instantly. Since the product is a solid, the absence of solvent results in the reaction producing a thick paste. To overcome this, a small quantity of EtOAc (5 mL) was added to form a slurry and prevent any detrimental blockages. Rotational speeds between 300–450 rpm, delivered the product after a residence time of 60 s, with filtration being the only purification required to give the aldehyde in 93% yield. This solid-state continuous oxidation presents advantages over solvent versions.39 Firstly, is the omission of DMSO, which is typically used in solvent protocols due to the established poor solubility of IBX. Secondly, the reaction proceeds much faster with completion after just 60 s compared to 12 hour reaction times typically seen in solution.
Metal-catalysed C–C and C–X coupling has recently been well explored under ball-milling conditions.40 Solid-state Sonogashira coupling has previously been achieved in batch regimes. Whilst continuous methods utilizing solid supports have been demonstrated, solid feeding of materials into a solvent-free continuous reactor would be beneficial. With this in mind, using their SSE technology (Scheme 8F), successful coupling was achieved with combined feeding of 2-iodothiophene and DABCO into one inlet and phenyl acetylene with Pd(OAc)2 into the other. The coupled product was achieved in 85% yield in 65 s residence time with a screw speed of 350–450 rpm. A reaction temperature of 0 °C was found to be optimal, which was suggested to temper reaction exothermicity, in turn reducing the formation of side products.
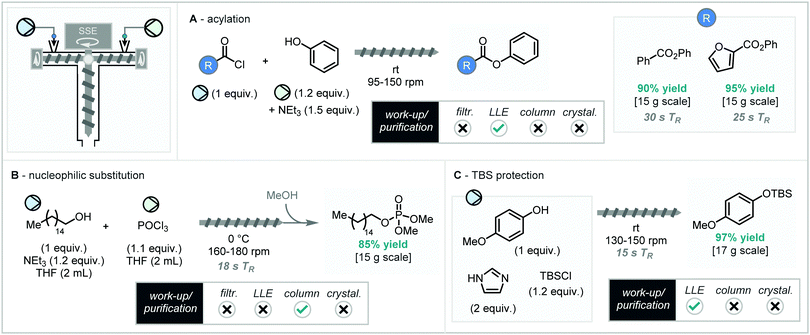
Using SSE, the acylation of phenol (in THF) with benzoyl chloride (in THF) in the presence of NEt3 proceeded efficiently to give the desired ester in 90% yield after a residence time of 25 s at 150–210 rpm (Scheme 9A). NEt3·HCl was produced as the by-product without hindering reactivity. It was found that 2-furoyl chloride could be used in place of benzoyl chloride giving the desired heteroaryl ester with a 95% yield after just 15 s residence time, also with no sign of blockage. This presents a beneficial alternative to solution-phase flow regimes for the continuous acylation of alcohols, as precipitation and blockage problems arising from salt formation are negated.
A similar approach can also be taken for nucleophilic substitution of POCl3 for the synthesis of biologically relevant organophosphorus compounds. Cetyl alcohol reacts with POCl3 in the presence of NEt3 at 0 °C and 160–180 rpm to produce the phosphonic dichloro-intermediate along with NEt3·HCl, before quenching the dichloro-intermediate with methanol after exit from the extruder to afford the product with a residence time of 18 s and a yield of 85% after column chromatography (Scheme 9B).
The final reaction reported using the SSE from Kulkarni and co-workers was the TBS-protection of phenols. 4-Methoxyphenyl, TBSCl and imidazole were directly added to the SSE at RT and 30–150 rpm. The silylether was produced after a 15 s residence time with a 94% yield (Scheme 9C). Again, an amine salt – in this case imidazole·HCl, was produced as a byproduct of the process in this reaction and seemingly did not hinder reactivity or operation of the extruder device.
3.7. James – 2020
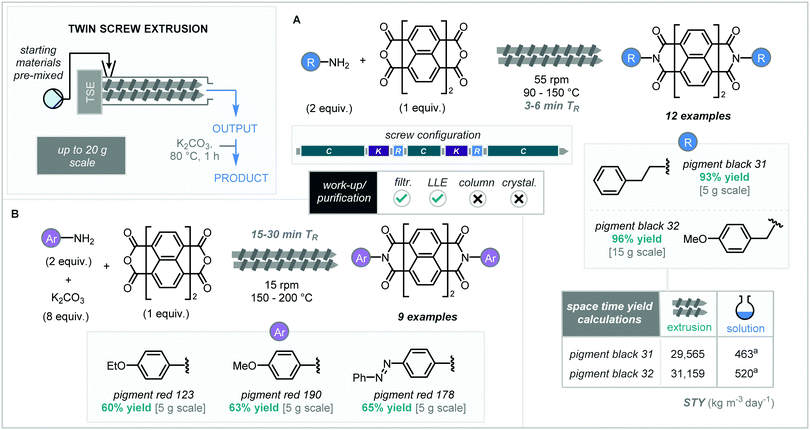
Organic dyes are widely utilised across many aspects of organic and material science, but issues arise upon their synthetic pathways. Conventional solution syntheses suffer from environmental setbacks due to contamination and the use of toxic reagents and/or solvents. This is highlighted in the synthesis of perylene-3,4,9,10-tetracarboxylic acid diimines (PDIs) where a large excess of amine and high boiling solvents (>160 °C) are required, often at high temperatures or in the presence of a promoter (i.e., zinc acetate).41 Indeed, several molecules used in materials applications also often suffer from solubility problems and have been a particular focus for the mechanochemistry community.42From a mechanochemical stand-point, James and co-workers have explored a more sustainable method for the synthesis of PDIs using solvent-free continuous extrusion technology (Scheme 10).43 Initial optimization began with solvent-free ball milling of 1,8-naphthalic anhydride with 4-ethoxyaniline using a ‘beat and heat’ method whereby the mixture is milled for 5 minutes before being heated to specific temperatures for 30 minutes. It was found that when temperatures below 110 °C were used the reaction does not proceed. However, at 110 °C quantitative conversion to product was observed.
Translation to continuous extrusion commenced with the manual introduction of 1,8-naphthalic anhydride and 4-ethoxyaniline into the extruder over 8 minutes at 55 rpm.
Aligned to the initial ball-milling studies, it was observed that no reaction occurred with barrel temperatures below 110 °C, whereas at 110 °C quantitative yield was again observed. This protocol was then extended to the reaction of perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA), providing the imminent precursor to many pigment dyes. When using PTCDA, the post extrusion materials were also then stirred in aqueous K2CO3 and held at 80 °C to hydrolyze any remaining starting material (Scheme 10). The use of PTCDA and two equivalents of the desired primary amine into the extruder afforded pigment black 31 and 32 in excellent yields (93% and 96% respectively) on a 5 g scale (Scheme 10A). Subsequent further scale up using automated feeders with PTCDA and the amine to feed into the TSE at rates of 0.65 g min−1 and 0.5 mL min−1 respectively. Hence, producing pigment black 31 and 32 on a 20 g scale with a 99% yield and a STY nearly 60 times that performed in solution, showcasing a solvent-free scalable continuous process towards high-value organic dyes. Other PDIs based on aniline-type amines were also synthesised but required alteration of the reaction conditions to compensate for the reduced nucleophilicity of the amine (Scheme 10B). Slower screw-rotational speeds of 15 rpm and increased temperatures between 150–200 °C along with a reduced feed rate (40–60 min) and the addition of K2CO3 afforded pigment red 123, 190 and 178 in good yields (60–65%).
3.8. El-Remaily – Jan 2020
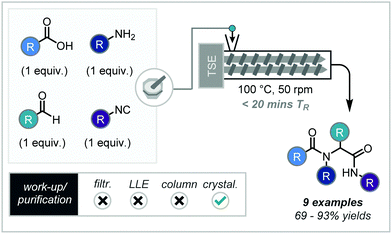
Multi-component reactions (MCR) – such as the Ugi reaction – provide unrivalled modular synthetic strategies towards biologically relevant compounds, due to atom economy, high efficiency, low cost, and simple experimental procedure.In 2020, a rapid solvent-free procedure was reported by El-Remaily and co-workers using a co-rotating extruder. The reagents were mixed in a glass mortar for an unspecified amount of time prior to addition to the LechTech Scientific 26 mm twin screw extruder, to ensure liquid reagent retention (Scheme 11).44
For optimization, the model system of benzaldehyde, aniline, benzoic acid and tert-butyl isocyanide was used. The highest yields were observed when water was used as a solvent (66%), however, the use of a solvent-less system resulted in a similar yield (63%). Heating of the extrusion system was shown to be key, with a reactor temperature of 100 °C accessing increased yields of 77%. Further to this, screw speeds were then lowered to 50 rpm to increase residence time of the material on the screw, resulting in yields of 93% of the product. A nine-compound scope followed optimisation with yields ranging from 69–93% and synthesised in under 20 minutes, demonstrating the reactions versatility to a variety of substituents. Furthermore, the products were obtained in high purity with the only purification requiring adding the extruded mixture to ice water and collecting the precipitate.
3.9. Crawford & Colacino – Aug 2020
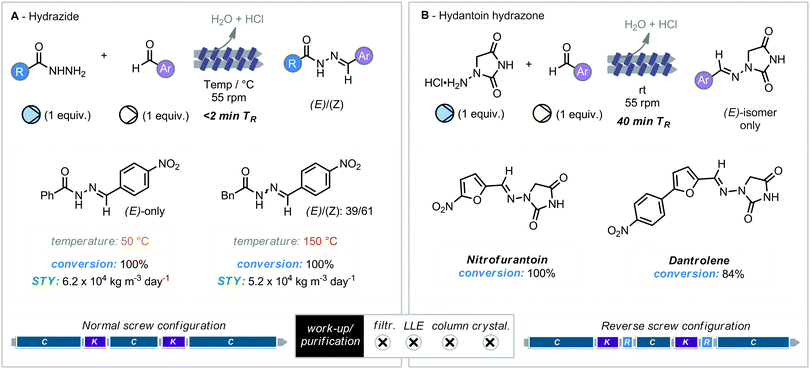
Reducing the overall environmental impact of the synthesis of active pharmaceuticals is a key aim of the ACS Green chemistry roundtable, who have set forth a range of key objectives by which this can be achieved.45 Among these aims include the reduction in the use of solvents and providing reactivity without the dependence on highly reactive or dangerous reagents. In 2020, Crawford, Colacino and co-workers’ reported the development of extrusion methodology towards the synthesis of hydantoin-based API's.46 Previous work from the Colacino group utilizing batch mode ball milling had established the synthesis of hydrazone containing pharmaceuticals, dantrolene (muscle relaxant) and nitrofurantoin (antibiotic), from 1-aminohydantoin via a base and solvent free protocol.47 Translation of this method to the continuous production of hydrazones through twin screw extrusion was initially achieved for model hydrazide compounds derived from acyl-hydrazine derivatives. Using a standard screw configuration, two derivatives were synthesized with complete conversion in under 2 minutes residence time. Interestingly only the (E)-isomer was observed with phenyl-substituted hydrazide. Further expansion of this methodology of this continuous synthesis protocol to the previously explored dantrolene and nitrofurantoin proved more challenging. Their synthesis required the alteration of the screw configuration to allow for increased residence time within the reactor (ca. 40 minutes), and only the (E)-isomer observed in both cases. Notably the construction of these pharmaceuticals could be carried out in an extruder without the use of concentrated mineral acids and bases, which have been previously required in solution. This consideration is further exemplified by the workup process, where the absence of acids and bases obviates the need for additional manual handling steps, although limited information is provided on the exact post-extrusion manipulations. A further study on this system by a consortium of mechanochemistry and extrusion experts truly highlights the energy consumption and water wastage benefits to extrusion methodology when compared to standard solution-phase set-ups for upscaling synthetic protocols (Scheme 12).484. “How-to” extrusion reaction design
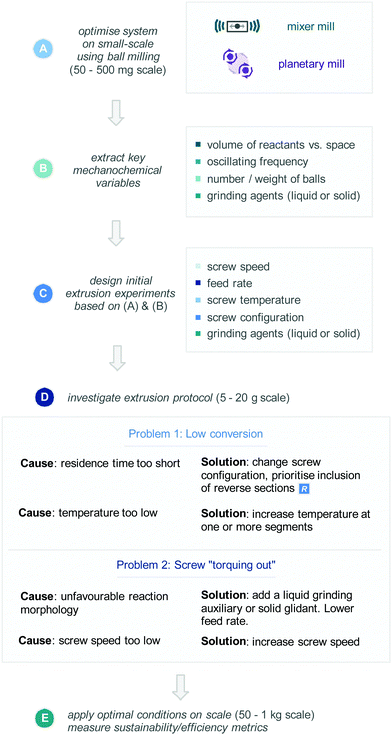
Whilst every reaction system contains its own subtleties, drawing knowledge from the case studies above and our own experience with the development of extrusion protocols allows us to form a general route for optimization of an extrusion process (Scheme 13).(A) Ball-milling optimization
Considering the large quantities of material required for each extrusion run (>10 g of total material), this could prove incredibly wasteful for studying an extrusion process on high-value intermediates. Accordingly, a large majority of the processes discussed above have been adapted from established ball-milling protocols. Small-scale optimization can be carried out using ball-milling apparatus on 50–500 mg scale, with either mixer mills or planetary mills. Incidentally shear mechanical forces predominate in the kneading sections of the extruder, therefore reactions that perform effectively in the planetary mill may be very well-placed for translation into extrusion protocols. These reactions will allow the user to optimise the reaction components (reagents, additives, catalysts, etc.), as well as reaction time required. Furthermore, unique ball-milling variables such as milling volumes, ball size/weight, liquid assisted grinding (LAG), grinding auxiliaries, and oscillating frequency will also be deduced.(B) Extract key variables
Information on reaction components and solid and liquid grinding auxiliaries should be directly applicable to extrusion method development. Although twin-screw extrusion offers a variety of new variables such as screw speed, feed rate, screw temperature, and screw configuration, which do not have direct comparisons in ball milling. Despite this, extracting the ball-milling-specific parameters coupled with reaction time can provide information of the amount of mechanical energy required for a transformation to take place, notably the “severity” of the reaction conditions. For example, if a reaction requires a heavy ball, high oscillating frequencies, and long reaction times in a ball-mill, it is likely that a high screw speed and residence time will be required in extrusion.(C) Design initial experiments
Using knowledge from sections (A) and (B), initial experiments can be designed. This is when automation capabilities are taken into account, i.e. whether a solid feeder/liquid pump can be used to accurately regulate feed rates of materials. In this case we would recommend starting at an addition rate at around 1–2 g min−1. If these are not available, one can manually add materials via a spatula or a syringe for initial experiments. Unless previous investigations demonstrated a need for harsh mechanochemical reaction conditions, we would recommend starting with a standard screw configuration (with no reverse sections), a screw speed of ∼50 rpm, and a screw temperature of 25 °C. However, as discussed above, the exact amount of material available for optimization in the extruder will govern how many experiments the user is able to conduct to discover an efficient process.(D) Investigate extrusion protocols
Initial experiments will provide a lot of information about reaction efficiency which can be judged via standard analytical techniques, such as thin layer chromatography (TLC), NMR, or LC-MS, but also by PXRD analysis of the crude extruded material vs. standards of the starting materials and products. Whilst there are many problems and causes held within the development of an extrusion protocol, we wish to highlight two routine problems, their common causes, and possible solutions.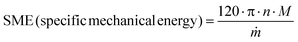 | (1) |
Whilst multiple variables will affect the residence time the most productive way to increase residence time is reconfiguration of the screws in the extruder. This can be done by incorporating reverse elements into the screw configuration, either through arrangement of 30° or 60° kneading sections in a reverse orientation, or through inclusion of reverse segments. Secondly, if low conversion is observed increasing the reaction temperature is a good option. Whilst increasing temperature of ball milling apparatus remains non-trivial,42 with extrusion apparatus it is more straightforward. Through simply increasing the temperature at one or more segments, one can supplement the mechanical energy imparted on the reagents with thermal energy.
(E) Apply the optimal conditions on higher scale
After optimisation of extruder parameters, running the continuous process for longer periods of time is critical to process success. This will provide information on reaction and reactor steady states, including long-term temperature and torque changes. Running in a continuous fashion will also allow the user to exemplify their protocol on industrially-relevant scales.5. Conclusions & future outlook
Building on pioneering work in crystal engineering and MOF synthesis (among many other areas), molecular synthetic organic strategies using reactive extrusion have begun to emerge in the last few years. Through elegant advances at the interface of chemical engineering and synthetic organic chemistry, twin-screw extrusion has placed itself as a unique technology in the processing of solvent-free and solvent-minimised reaction systems.Twin-screw extruders have been shown to enable fine-tuning of new reaction parameters such as screw speed and feed rates, but also allowing in the complete modulation of screw configurations and modular temperature control of separate sections. Altogether this has provided a programmable framework for continuous mechanochemistry. Further to this, this concept has enabled the showcasing of alternative screw systems such as single screw extruders, and recirculating screw extruders. Using extrusion, a variety of transformations have been achieved including condensations, palladium-catalysed cross couplings, Michael additions, and oxidative dimerization methods.
This tutorial review has covered the first landmark papers in this field and hopes to stimulate further research in the area by spotlighting the opportunities reactive extrusion can offer both sustainable and continuous chemistry. The future outlook for exploring and implementing reactive extrusion for organic synthesis is rather exciting. There are a myriad of reactions that remain to be explored and developed, including several key reactions currently applied at high volume with vast amounts of solvent consumed. Caution of course needs to be applied to potential safety hazards for processing materials in this manner, avoid anything that could be shock sensitive or rapidly release gases, and perform appropriate safety tests prior to commencing work. Exploring the use of hybrid continuous reactors consisting of solid and liquid flow setups to implement multi-step chemical synthesis offers a particularly captivating prospect. Still, much work needs to be investigated and recorded on optimising and implementing the use of extruders and their variety of parameters as well as focus on optimising the purification of these processes for maximum purity, and both minimising the amount of solvent used and number of manual handling steps required. Furthermore, the translation from ball-milling to reactive extrusion is often not straightforward, and we hope this review may act as a starting point guide for end users looking to engage in continuous mechanochemistry, and the wealth of opportunities this currently under-utilised technique has to offer.49
Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. R. A. B. thanks UCL for a studentship. J. A. L. thanks the Leverhulme trust for a research fellowship (RPG-2019-260). D. L. B. is grateful to the European Union, WEFO, Cardiff University and Cambridge Reactor Design for a Knowledge Economy and Skills Scholarship (KESS2) to A. C. J. W. I. N. thanks Cardiff University for a studentship. The authors thank Margharete Richter (Thermofisher) for valuable discussions.Notes and references
- M. Baumann, T. S. Moody, M. Smyth and S. Wharry, Org. Process Res. Dev., 2020, 24, 1802–1813 CrossRef CAS.
- M. Movsisyan, E. I. P. Delbeke, J. K. E. T. Berton, C. Battilocchio, S. V. Ley and C. V. Stevens, Chem. Soc. Rev., 2016, 45, 4892–4928 RSC.
- D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276–10341 CrossRef PubMed.
- M. Brzozowski, M. O’Brien, S. V. Ley and A. Polyzos, Acc. Chem. Res., 2015, 48, 349–362 CrossRef CAS PubMed.
- M. B. Plutscack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796–11893 CrossRef PubMed and references therein.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC.
- M. Guidi, P. H. Seeberger and K. Gilmore, Chem. Soc. Rev., 2020, 49, 8910–8932 RSC.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Sharouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef CAS.
- K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025–1074 CrossRef CAS PubMed.
- L. Takacs, Chem. Soc. Rev., 2013, 42, 7649–7659 RSC.
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC and references therein.
- K. J. Ardila-Fierro and J. G. Hernández, ChemSusChem, 2021, 14, 2145–2162 CrossRef CAS PubMed.
- K. Kubota, R. Takahashi and H. Ito, Chem. Sci., 2019, 10, 5837–5842 RSC.
- J. L. Howard, M. C. Brand and D. L. Browne, Angew. Chem., Int. Ed., 2018, 57, 16104–16108 CrossRef CAS PubMed.
- For reviews containing key references not related to synthetic organic chemistry see: (a) D. E. Crawford, Beilstein J. Org. Chem., 2017, 13, 65–75 CrossRef CAS PubMed; (b) D. E. Crawford and J. Casaban, Adv. Mater., 2016, 28, 5747–5754 CrossRef CAS PubMed.
- F. Gomollón-Bel, Chem. Int., 2019, 41, 12–17 Search PubMed.
- D. E. Crawford, S. L. James and T. McNally, ACS Sustainable Chem. Eng., 2018, 6, 193–201 CrossRef CAS.
- D. E. Crawford, C. K. G. Miskimmin, A. B. Albadarin, G. Walker and S. L. James, Green Chem., 2017, 19, 1507–1518 RSC.
- D. Stan, M. Matache, L. Ruta, C. Draghici and C. Dobrota, Rev. Chim., 2009, 60, 876–879 CAS , https://bch.ro/pdfRC/STAN%20DANA%209.pdf.
- D. E. Crawford, C. K. G. Miskimmin, A. B. Albadarin, G. Walker and S. L. James, Green Chem., 2017, 19, 1507–1518 RSC.
- G. Kaupp, M. R. Naimi-Jamal and J. Schmeyers, Chem. – Eur. J., 2002, 8, 593–600 Search PubMed.
- K. Gilmore, S. Vukelić, T. McQuade, B. Koksch and P. H. Seeberger, Org. Process Res. Dev., 2014, 18, 1771–1776 CrossRef CAS.
- V. Isoni, K. Mendoza, E. Lim and K. Teoh, Org. Process Res. Dev., 2017, 21, 992–1002 CrossRef CAS.
- F. Faigl, A. Thurner, B. Molnár, G. Simig and B. Volk, Org. Process Res. Dev., 2010, 14, 617–622 CrossRef CAS.
- S. Senaweera and J. D. Weaver, Chem. Commun., 2017, 53, 7545–7548 RSC.
- D. E. Crawford, C. K. Miskimmin, J. Cahir and S. L. James, Chem. Commun., 2017, 53, 13067–13070 RSC.
- K. L. Kirk, J. Fluorine Chem., 2006, 127, 1013–1029 CrossRef CAS.
- J. L. Howard, Y. Sagatov, L. Repusseau, C. Schotten and D. L. Browne, Green Chem., 2017, 19, 2798–2802 RSC and citing articles.
- Q. Cao, J. L. Howard, D. E. Crawford, S. L. James and D. L. Browne, Green Chem., 2018, 20, 4443–4447 RSC.
- V. Declerck, P. Nun, J. Martinez and F. Lamaty, Angew. Chem., Int. Ed., 2009, 48, 9318–9321 CrossRef CAS PubMed.
- K. J. Ardila-Fierro, D. E. Crawford, A. Körner, S. L. James, C. Bolm and J. G. Hernández, Green Chem., 2018, 20, 1262–1269 RSC.
- Y. Yeboue, B. Gallard, N. Le Moigne, M. Jean, F. Lamaty, J. Martinez and T.-X. Métro, ACS Sustainable Chem. Eng., 2018, 6, 16001–16004 CrossRef CAS.
- B. M. Sharma, R. S. Atapalkar and A. A. Kulkarni, Green Chem., 2019, 21, 5639–5646 RSC.
- F. Toda, K. Tanaka and S. Iwata, J. Org. Chem., 1989, 54, 3007–3009 CrossRef CAS.
- C. Kuhakarn, K. Kittigowittana, P. Ghabkham, M. Pohmakotr and V. Reutrakul, Synth. Commun., 2006, 36, 2887–2892 CrossRef CAS.
- M. Gholinejad, F. Hamed and P. Biji, Dalton Trans., 2015, 44, 14293–14303 RSC.
- T. Sugino and K. Tanaka, Chem. Lett., 2001, 110–111 CrossRef CAS.
- K. Surendra, N. S. Krishnaveni, M. A. Reddy, Y. V. D. Nageswar and K. R. Rao, J. Org. Chem., 2003, 68, 2058–2059 CrossRef CAS PubMed.
- A. Porcheddu, E. Colacino, L. De Luca and F. Delogu, ACS Catal., 2020, 10, 8344–8394 CrossRef CAS and references therein.
- H. E. Katz, A. J. Lovinger, J. Johnson, C. Kloc, T. Siegrist, W. Li, Y. Y. Lin and A. Dodabalapur, Nature, 2000, 404, 478–481 CrossRef CAS PubMed.
- T. Seo, N. Toyshima, K. Kubota and H. Ito, J. Am. Chem. Soc., 2021, 143, 6165–6175 CrossRef CAS PubMed.
- Q. Cao, D. E. Crawford, C. Shi and S. L. James, Angew. Chem., Int. Ed., 2020, 59, 4478–4483 CrossRef CAS PubMed.
- M. A. E. A. A. A. El-Remaily, A. M. M. Soliman and O. M. Elhady, ACS Omega, 2020, 5, 6194–6198 CrossRef.
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer, R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411–420 RSC and references therein.
- D. E. Crawford, A. Porcheddu, A. S. McCalmont, F. Delogu, S. L. James and E. Colacino, ACS Sustainable Chem. Eng., 2020, 8, 12230–12238 CrossRef CAS.
- E. Colacino, A. Porcheddu, I. Halasz, C. Charnay, F. Delogu, R. Guerra and J. Fullenwarth, Green Chem., 2018, 20, 2973–2977 RSC.
- O. Galant, G. Cerfeda, A. S. McCalmont, S. L. James, A. Porcheddu, F. Delogu, D. E. Crawford, E. Colacino and S. Spatari, ACS Sustainable Chem. Eng., 2022, 10, 1430–1439 CrossRef CAS.
- During final preparations of this review Andersen and co-workers reported a twin-screw enabled SNAr reaction including a neat demonstration of conversion from a heated mill to an extruder device, see: J. Andersen, H. Starbuck, T. Current, S. Martin and J. Mack, Green Chem., 2021, 23, 8501–8509 RSC.
| This journal is © The Royal Society of Chemistry 2022 |