Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insights into the role of molar ratio and added water in the properties of choline chloride and urea-based eutectic mixtures and their cellulose swelling capacity†
Juho Antti
Sirviö
 *a,
Riikka
Haataja
a,
Anu M.
Kantola
*a,
Riikka
Haataja
a,
Anu M.
Kantola
 b,
Terhi
Suopajärvi
a and
Henrikki
Liimatainen
b,
Terhi
Suopajärvi
a and
Henrikki
Liimatainen
 a
a
aFibre and Particle Engineering Research Unit, University of Oulu, P.O. Box 4300, 90014 Oulu, Finland. E-mail: juho.sirvio@oulu.fi
bNMR Research Unit, University of Oulu, P.O. Box 3000, FIN-90014, Oulu, Finland
First published on 10th November 2022
Abstract
Eutectic mixtures and deep eutectic solvents (DESs) are promising green media for the pre-treatment of lignocellulose materials. They can be harnessed for the swelling of cellulose and further facilitate cellulose hydrolysis, derivatization, and production of cellulose-based (nano) materials. Several studies indicated that water can take part in the formation of the nanostructure of DES; however, it is still unclear how additional water influences many important properties and functioning of DES, especially when the molar ratio of compounds differs from the eutectic point composition. Here, viscosity, pH, conductivity, solvatochromic and solvatomagnetic solvent parameters, and fiber swelling capacity of choline chloride and urea mixtures demonstrating different molar ratios were investigated in the presence and absence of added water. The participation of water in the formation of molecular clusters with choline chloride and urea was indicated by viscosity, pH, and conductivity measurements. Hydrogen bond acceptor values of aqueous mixtures increased as a function of water content, and the results obtained using both methods were in line, indicating their suitability for the determination of hydrogen bond acidity of aqueous choline chloride–urea mixtures. However, hydrogen bond basicity determined by solvatochromic and magnetic methods exhibited almost opposite trends. The close investigation of the chemical shift of solvatomagnetic probes indicated that the chemical environment of the choline chloride–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture remained constant until the water content of 30 wt% was in line with previous molecular simulations. When cellulose fibers were treated with mixtures under mixing, the non-ideality of the choline chloride–urea mixture and the absence of water were found to be advantageous; however, aqueous mixtures efficiently increased the diameters of cellulose fibers in the absence of mixing, and water-containing mixtures appeared to be appealing systems for cellulose pretreatments.
2) mixture remained constant until the water content of 30 wt% was in line with previous molecular simulations. When cellulose fibers were treated with mixtures under mixing, the non-ideality of the choline chloride–urea mixture and the absence of water were found to be advantageous; however, aqueous mixtures efficiently increased the diameters of cellulose fibers in the absence of mixing, and water-containing mixtures appeared to be appealing systems for cellulose pretreatments.
Introduction
Eutectic mixtures and deep eutectic solvents (DESs) are a versatile group of chemical systems used in numerous potential applications, such as catalysts and extraction and modification of metals and biomaterials.1 DESs can be described as eutectic mixtures with eutectic point temperature lower than their corresponding ideal liquid mixtures.2 The formation of DES is generally assumed to be due to the strong hydrogen bond interaction between constituents, that is, hydrogen bond donor(s) and acceptor(s), although other interactions such as van der Waals forces can also contribute to DES formation.3 The strong interaction between DES compounds prevents the crystallization of individual chemicals, thus depressing the eutectic point temperature.DESs can be easily produced from a wide range of chemicals, including ones with low toxicity and good biodegradability, although a direct conclusion on DES characteristics cannot be made based on DES starting materials4,5 For example, DES of choline chloride and urea exhibits low toxicity, and is readily biodegradable,6 and is widely recognized as “architype” DES.
DESs are promising materials in various fields, including nanoscience.7 One of the potential applications is the production of cellulose nanomaterials.8–11 Due to the recalcitrant structure of natural cellulose fibers, the liberation of nano-sized cellulose constituents is a highly energy-consuming process, thus decreasing the environmental and economic feasibility of the processing.12 DESs can be used to swell cellulose fibers without notable chemical modification,13–16 enabling the production of cellulose nanomaterials with lower energy consumption.17 Also, DESs and eutectic mixtures can be used for the chemical modification of cellulose fibers and wood to produce cellulose derivatives and nanomaterials with different surface functionalities.18–23
Cellulose and many DESs are highly hygroscopic materials, and a certain amount of water is always present in the mixtures.24 Complete drying of cellulose and DES prior to their use and during solvent recycling requires extra energy, and thus, aqueous DES could enable more straightforward cellulose processing.14 In addition, due to the many positive characteristics, water can be used as a potential co-solvent25 or even part of the DES system.26 However, water is an amphiprotic molecule and can thus notably alter the properties of DES.27 For example, water can form competing hydrogen bonds and change the interactions between DES and cellulose fibers. Furthermore, water can alter the polarity of solvents and the bonding between DES and amphiphilic cellulose. In addition, water can alter the dissolution properties of DES, and multicomponent solvents formed between DES and water could be used in areas other than cellulose processing. For example, xylan was found to be highly soluble in 50 wt% aqueous choline chloride–urea solution.28
Several studies reported the properties of aqueous DESs (especially in the case of choline chloride–urea).27,29–32 The added water can take part in the molecular structure of DES, yet an increase in the amount of water beyond a certain limit, around 50 wt% in the case of choline chloride–urea, leads to changes in the structure of the DES complex, and the mixture behaves like a water solution of its individual compounds.33 However, how the addition of water alters many important properties of DES is still poorly understood, especially when different molar ratios of DES compounds are used (i.e., when a ternary system is formed at different molar ratios34). Here, choline chloride–urea mixtures with molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were prepared in the presence and absence of added water (maximum water dosage of around 50 wt%), and their viscosity, pH, and conductivity were investigated. Furthermore, polarity (ENT), dipolarity/polarizability (π*), and hydrogen bond acceptor (α) and donor (β) properties were determined using the solvatochromic method. Also, the α and β parameters were revealed with the solvatomagnetic method, and results obtained using these two approaches were compared. Finally, the swelling of cellulose fibers in different choline chloride–urea mixtures were investigated to elucidate the role of the molar ratio and the added water in the alteration of cellulose fiber morphology.
1 were prepared in the presence and absence of added water (maximum water dosage of around 50 wt%), and their viscosity, pH, and conductivity were investigated. Furthermore, polarity (ENT), dipolarity/polarizability (π*), and hydrogen bond acceptor (α) and donor (β) properties were determined using the solvatochromic method. Also, the α and β parameters were revealed with the solvatomagnetic method, and results obtained using these two approaches were compared. Finally, the swelling of cellulose fibers in different choline chloride–urea mixtures were investigated to elucidate the role of the molar ratio and the added water in the alteration of cellulose fiber morphology.
Materials and methods
Materials
Choline chloride (98.0%) and urea (99.0–100.5%) were obtained from Algry Quimica S. L. and Sigma-Aldrich, respectively. The pH of the solvents was adjusted with 0.1 M sodium hydroxide from FF Chemicals. Softwood Kraft pulp fibers used in the swelling experiments were obtained from MetsäFibre, Finland. Deionized water was used throughout the experiments. Probes used in solvatochromic measurements were 4-nitroaniline (≥99%) (NA) from Sigma-Aldrich, N,N-diethyl-4-nitroaniline (98%) (DENA) from Apollo Scientific Ltd, and Nile Red from Tokio Chemical Industry Co. The reference solvents were dimethyl sulfoxide (>99.0%) from GC Chemicals and cyclohexane (99%) from RCI Labscan. Probes used in solvatomagnetic measurements were 4-fluoroanisole (≥97.0%, GC Chemicals), 4-fluorophenol (>99.0%, Tokyo Chemical Industry Co.), and pyridine-N-oxide (95%, Sigma-Aldrich). Deuterated chloroform with 0.03% tetramethylsilane (99.80% D, Eurisotop) and trifluoroacetic acid (>99.0%, Tokyo Chemical Industry Co.) were used as internal standards. The chemical structure of the compounds used to produce solvent systems as well as used probes in solvatochromic and solvatomagnetic experiments are presented in Fig. S1 (ESI†).Preparation of choline chloride–urea mixtures with variable molar ratios and amount of added water
Pure mixtures of choline chloride and urea corresponding deep eutectic and non-eutectic point compositions were prepared by mixing the compounds at molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, followed by heating in an oven at 100 °C for 20 h in a closed Scott bottle. Next, mixtures with added water were prepared by the addition of water into the above-mentioned choline chloride–urea mixture at choline chloride–water molar ratios of 1
1, followed by heating in an oven at 100 °C for 20 h in a closed Scott bottle. Next, mixtures with added water were prepared by the addition of water into the above-mentioned choline chloride–urea mixture at choline chloride–water molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, and 1
8, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (12–51 wt% of whole mixture). The samples were named as CCUxy-z where x and y describe the molar ratio between choline chloride and urea, and z is the moles of added water per mole of choline chloride. As references, solutions of choline chloride and water at molar ratio of 1
10 (12–51 wt% of whole mixture). The samples were named as CCUxy-z where x and y describe the molar ratio between choline chloride and urea, and z is the moles of added water per mole of choline chloride. As references, solutions of choline chloride and water at molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (RefCC), urea and water at a molar ratio of 2
2 (RefCC), urea and water at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (RefUrea), pure water (RefWater), and RefCC and RefWater adjusted to pH 10 were used. The constitutions and namings of the samples are presented in Table 1.
1 (RefUrea), pure water (RefWater), and RefCC and RefWater adjusted to pH 10 were used. The constitutions and namings of the samples are presented in Table 1.
| Sample name | Percentage of compounds (wt%) | ||
|---|---|---|---|
| Choline chloride | Urea | Water | |
| CCU12-0 | 53.75 | 46.25 | 0 |
| CCU12-2 | 47.21 | 40.62 | 12.18 |
| CCU12-4 | 42.09 | 36.21 | 21.70 |
| CCU12-6 | 37.97 | 32.66 | 29.37 |
| CCU12-8 | 34.58 | 29.75 | 35.67 |
| CCU12-10 | 31.75 | 27.32 | 40.93 |
| CCU11-0 | 69.92 | 30.08 | 0 |
| CCU11-2 | 59.24 | 2548 | 15.27 |
| CCU11-4 | 51.39 | 22.11 | 26.50 |
| CCU11-6 | 45.38 | 19.52 | 35.10 |
| CCU11-8 | 40.63 | 17.48 | 41.90 |
| CCU11-10 | 39.77 | 15.82 | 47.41 |
| CCU21-0 | 82.30 | 17.70 | 0 |
| CCU21-2 | 67.89 | 14.60 | 17.51 |
| CCU21-4 | 57.78 | 12.43 | 29.80 |
| CCU21-6 | 50.29 | 10.82 | 38.90 |
| CCU21-8 | 44.51 | 9.57 | 45.92 |
| CCU21-10 | 39.93 | 8.59 | 51.48 |
| RefCC | 79.50 | 0 | 20.50 |
| RefUrea | 0 | 62.52 | 37.48 |
| RefWater | 0 | 0 | 100 |
| RefWater (pH 10) | 0 | 0 | 100 |
| RefCC (pH 10) | 79.50 | 0 | 20.5 |
Viscosity, pH, and conductivity of choline chloride–urea mixtures with various amounts of added water
The viscosity of solutions was measured using the Discovery HR-1 hybrid rheometer, TA Instruments, with a flow sweep procedure and a cone diameter of 40 mm and cone-plate angle of 1.999° and step time of 35 s. Next, all the mixtures with added water (except RefUrea, which is solid at room temperature) were measured at 20 °C, while CCU12-0 and CCU11-0 (melting point of appr. 80 °C under moisture-free conditions35) were measured at a temperature of 100 °C.Then, pH and conductivity were measured at a temperature of 23 °C, expect for e RefUrea, which was measured at 50 °C. CCU11-0 and CCU21-0 could not be measured due to the limitation of the temperature range of pH and conductivity devices. Both pH and conductivity were measured using an Accumet model 20 pH/conductivity meter.
Measurement of solvent parameters of choline chloride–urea mixtures with various amounts of added water
The polarity parameter ENT, the dipolarity/polarizability parameter π*, hydrogen-bond basicity (βC), and acidity (αC) were calculated using eqn (1),36 2,37 3,37 and 4,38 respectively:
ENT = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 591.44/λmax 591.44/λmax | (1) |
 | (2) |
| Δν = ΔνN,N − Δν4NA | (3) |
 | (4) |
Solvatomagnetic method
Nuclear magnetic resonance (NMR) measurements were performed on a Bruker Avance III 600 spectrometer. Spectra were recorded with a single pulse sequence and waltz proton decoupling during the acquisition. Referencing for all measurements were made at 25 °C.For the measurement of hydrogen bond acidity (αM), 47.55 mg of pyridine-N-oxide was dissolved into 2 mL solvent in an oven at 40 °C for 12 h. CCU12-0 was further heated for an additional 2 hours at 80 °C to fully dissolve pyridine-N-oxide. Next, for 13C measurements, a 30° pulse was used, spectral width was 240 ppm, relaxation delay was 2 s, and the number of scans was 128. Deuterated chloroform with tetramethylsilane was used as an internal standard for 25 °C measurements (TMS = 0 ppm) and deuterated chloroform for 100 °C measurements (chloroform 77.16 ppm). The chemical shifts of C2 (δ2) and C4 (δ4) were recorded, and the αM was calculated using eqn (5):37,39
| αM = −0.15 × d24 + 2.32 | (5) |
The hydrogen bond basicity (βM) was measured by preparing individual solutions containing either fluorophenol or fluoroanisol. Both probes were mixed individually with mixtures at a concentration of 1 mg mL−1. Fluoroanisol was dissolved at room temperature, whereas fluorophenol solutions were heated in an oven at 80 °C for 12 h, to obtain complete dissolution. In 19F measurements, a 90° pulse was used, spectral width was 30 ppm, relaxation delay was 10 s, and the number of scans was 64. Trifluoroacetic acid (76.55 ppm) was used as an internal standard. The βM was calculated using eqn (6):40
 | (6) |
Treatment of cellulose fibers with choline chloride–urea mixtures with various amounts of added water
A precalculated amount of choline chloride, urea, and water was weighed in a 250 mL Schott bottle. Next, the bottle was closed with a cap to minimize the evaporation of water, placed in an oil bath at 100 °C, and the mixture was mixed with a magnetic stirring bar until a clear liquid was formed (no clear liquid was formed in case of CCU21-0). Dry sheets of cellulose were torn into smaller pieces (around 1 × 1 cm) by hand and added to the mixture at a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid mass ratio of 1
liquid mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and mixed for one hour. Then, 20 mL of water was added, and the suspension was filtered and washed with 1000 mL of water. Samples were stored at 4 °C. Reference samples without any mixing treatment were prepared in a similar manner excluding the mixing step after the addition of cellulose.
50 and mixed for one hour. Then, 20 mL of water was added, and the suspension was filtered and washed with 1000 mL of water. Samples were stored at 4 °C. Reference samples without any mixing treatment were prepared in a similar manner excluding the mixing step after the addition of cellulose.
Fiber dimensions of the original and treated cellulose samples were analyzed using a Valmet FS5 image analyzer. The analysis was conducted as triplicates, and the results were averaged. A reference sample analysis of non-treated pulp was prepared according to the ISO5263-1:2004I standard.
Results and discussion
Appearance and viscosity of choline chloride–urea mixtures
The choline chloride–urea mixture at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (sample CCU12-0) has often been considered to represent the eutectic point composition (i.e., true DES) exhibiting a melting point notably lower (the lowest melting point, i.e., eutectic point temperature in the phase diagram) compared to either starting materials and their predicted ideal mixture. The behavior of the synthesized solvents supported this definition, as CCU12-0 was the only sample that remained liquid at room temperature without the addition of water. However, after several months of storage, CCU12-0 slowly crystallized.
2 (sample CCU12-0) has often been considered to represent the eutectic point composition (i.e., true DES) exhibiting a melting point notably lower (the lowest melting point, i.e., eutectic point temperature in the phase diagram) compared to either starting materials and their predicted ideal mixture. The behavior of the synthesized solvents supported this definition, as CCU12-0 was the only sample that remained liquid at room temperature without the addition of water. However, after several months of storage, CCU12-0 slowly crystallized.
The melting point of the DES 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of choline chloride and urea has been reported to be below room temperature (12 °C).41 However, the reported low melting point of choline chloride–urea can be due to the presence of a minor amount of water (due to the hygroscopicity of the two chemicals24), and the melting point of 31.8 °C has been reported for CCU12-0 after the extensive drying step.35 Here, we did not use any additional drying step, and mixtures are expected to contain a minor amount of water, supported by the presence of a small peak of water in the 1H NMR spectrum of CCU12-0 (Fig. S2, ESI†).
2 mixture of choline chloride and urea has been reported to be below room temperature (12 °C).41 However, the reported low melting point of choline chloride–urea can be due to the presence of a minor amount of water (due to the hygroscopicity of the two chemicals24), and the melting point of 31.8 °C has been reported for CCU12-0 after the extensive drying step.35 Here, we did not use any additional drying step, and mixtures are expected to contain a minor amount of water, supported by the presence of a small peak of water in the 1H NMR spectrum of CCU12-0 (Fig. S2, ESI†).
CCU11-0 formed a liquid when heated to 100 °C, but the mixture solidified when cooled to room temperature. Conversely, CCU21-0 was a highly viscous and turbid mixture even when heated to 100 °C for 24 h. In addition, RefUrea remained a supercooled liquid at room temperature, and it solidified when mixed. All the other water-containing samples were liquid at room temperature.
At room temperature, all water-containing choline chloride–urea samples exhibited a strong shear thinning (non-Newtonian) property, that is, their viscosity decreased with the increase in the shear rate (Fig. 1). The shear thinning is most notable at a shear rate from 0.1 to 1 s−1 (insert in Fig. 1), after which the viscosities of all samples remained at a similar level (viscosities of all samples at a shear rate of 10 s−1 were in the range of 1.16–1.71 Pa s). The non-Newtonian viscosity is in agreement with the previous observation of acid–based DESs.42 Shear thinning behavior has also been reported with some ionic liquids, particularly those demonstrating free amine, thiol, or hydroxyl groups, which can act as hydrogen bond donors and acceptors.43 In the case of the ionic liquid, the shear thinning is assumed to originate from the formation of molecular aggregates, for example, via hydrogen bonding. By applying an external force, these aggregates are disintegrated, resulting in a decrease in viscosity, as molecules are oriented along the shear direction.
In the case of ionic liquids, the addition of water (around 10 wt%) resulted in changes of non-Newtonian viscosity to Newtonian as the viscosity was observed to be independent of the shear rate.43 Here, all the studied water-containing choline chloride–urea systems demonstrated higher water contents (minimum added water content was 12 wt%), yet the shear thinning behavior was observed even with the sample containing 51 wt% of water (CCU21-10). Therefore, viscosity results indicated that the added water does not merely dilute the choline chloride–urea systems, but becomes a part of the molecular clusters, as has been proposed previously.33,44,45 Furthermore, it is notable that viscosity values do not decrease as a function of added water, despite some previous studies showing that the viscosity of choline chloride–urea (molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) decreases at the function of added water.46 The difference from the current study might originate from the use of a different measurement type (cone-plate rheometer vs. rolling-ball microviscometer). For example, at all choline chloride–urea molar ratios, the highest viscosity at a shear rate of 0.1 s−1 was found when 4 mol of water was added per mol of choline chloride (water content of 22–30 wt%). When the shear rate increased, the differences between samples decreased, and at a high shear rate, the differences in viscosities were negligible.
2) decreases at the function of added water.46 The difference from the current study might originate from the use of a different measurement type (cone-plate rheometer vs. rolling-ball microviscometer). For example, at all choline chloride–urea molar ratios, the highest viscosity at a shear rate of 0.1 s−1 was found when 4 mol of water was added per mol of choline chloride (water content of 22–30 wt%). When the shear rate increased, the differences between samples decreased, and at a high shear rate, the differences in viscosities were negligible.
It is also noteworthy that the RefCC sample, which contained only choline chloride and water, exhibited strong shear thinning behavior (Fig. S3a, ESI†), and the viscosity values were similar to those observed with water-containing choline chloride–urea systems. The shear thinning of RefCC indicates that molecular aggregates were also formed between pure choline chloride and water. Water forms a eutectic mixture with NaOH47 as well as dimethyl sulfoxide,48 and the mixture between choline chloride and water may also behave as a kind of eutectic solvent. Recently, the mixture of choline chloride and water at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.33 has been described as DES, where water acts as a hydrogen bond donor.49 Furthermore, the solid-like behavior of the 14N to β-CH (–CH2–O–) coupling determined by NMR led to the assumption of the formation of a semi-rigid framework clathrate structure of choline chloride (and other halide ions) at a low level of hydration (i.e., at a small amount of added water).50
3.33 has been described as DES, where water acts as a hydrogen bond donor.49 Furthermore, the solid-like behavior of the 14N to β-CH (–CH2–O–) coupling determined by NMR led to the assumption of the formation of a semi-rigid framework clathrate structure of choline chloride (and other halide ions) at a low level of hydration (i.e., at a small amount of added water).50
Despite being liquid at room temperature, CCU12-0 was too viscous to be measured at room temperature with the used measurement setup, and viscosity was, therefore, measured at 100 °C (Fig. S3b, ESI†). CCU11-0 was liquid at an elevated temperature, and its viscosity was also measured at 100 °C; however, due to the high viscosity and the presence of solid particles, CCU21-0 could not be measured even at 100 °C. The viscosity of DESs decreases drastically with the increase in temperature,46 and at 100 °C, CCU12-0 and CCU11-0 showed lower viscosity values compared to water-containing mixtures at room temperature. Especially, the effect of elevated temperature is notable at high shear rates. For example, at room temperature, the viscosity of CCU12-2 was 1.4 Pa s at a shear rate of 10 s−1, whereas at the same shear rate, the viscosity of CCU12-0 was 0.2 Pa s. At the low shear rate, CCU11-0 with higher urea content showed lower viscosity values, which is in line with computed viscosities,51 yet at a higher shear rate, no difference was found between the two samples.
pH and conductivity of choline chloride–urea mixtures
The pHs of all choline chloride–urea mixtures and RefUrea were found to be alkaline (from 9.27 of RefUrea to 10.97 of CCU12-0), whereas the pH of RefCC was slightly acidic (Fig. 2a). The pH values of water-containing samples are in line with the previous studies.28 Alkaline pH indicates that the pH of choline chloride–urea systems is due to the presence of urea. Urea is a very weakly basic molecule and exists mainly as a neutral molecule in water, yet the oxygen atoms of urea can act as basic sites52 (urea can even form a salt with strong acid). However, it is noteworthy that urea53 and choline chloride–urea DES35 are thermally labile compounds, and the degradation of urea into basic ammonium and carbon dioxide might also occur. Conversely, the quaternary ammonium group of choline chloride is a salt of a strong acid and base and is thus mainly neutral, while the slightly acidic pH of RefCC is due to the mild acidity of the hydroxyl group.54 | ||
| Fig. 2 (a) pH and (b) conductivity of the choline chloride–urea mixtures and their individual components as a function of water at room temperature (conductivity of RefUrea was measured at 50 °C). | ||
In all the different molar ratios between choline chloride and urea, the addition of water decreased the pH value, which was due to the decrease in the concentration of compounds (mainly urea). However, when the molar ratio between choline chloride and urea increases, the pH increases, and this effect is most clearly observed at lower water content. For example, the pH values of CCU12-2, CCU11-2, and CCU21-2 were 10.05, 10.10, and 10.25, respectively, whereas the urea contents were 40.62, 27.58, and 14.60 wt%, respectively. In addition, the pH of RefUrea, with the highest urea content, was lower compared to any choline chloride–urea mixtures. It is plausible that the degradation of urea54 is accelerated in the presence of choline chloride, however, it has been previously shown that a non-ideal mixture with high choline chloride content exhibited higher thermal stability than a mixture with the ideal molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).35
2).35
The possibility exists that the hydrogen bonding interaction between urea and choline chloride increases the basicity of urea. Researchers proposed that the main interaction in the choline chloride–urea system is between protons of amide (–NH2) groups of urea and chloride ions of choline chloride.55 The interaction of negatively charged chloride ion and urea can lead to the increase of electron density of the urea molecule, thus making it more basic (i.e., a better acceptor for proton). Previously, studies have shown that hydrogen bonding with dimethyl sulfoxide increases the basicity of water.56 Additionally, the increase in chloride ion concentration in choline chloride–urea systems increases the probability of interaction between urea and chloride ion, thus increasing the overall basicity of the systems, despite the decrease in urea content.
The increase in the water content demonstrated a clear trend in the increase in the conductivity of the mixtures (Fig. 2b). Previously, researchers postulated that the decrease of viscosity significantly contributes to the increase of conductivity in choline chloride–urea DES.57 Lower viscosity facilitates the mass transfer and thus fluidity of the solvent, which increases the conductivity. However, in the previous study, the maximum water content studied was 6 wt%, which is well below the minimum added water content used in the present work (12.70 wt% in the case of DES12-2). The decrease in the viscosity was significant at low water content (from 1080 mPa s of pure DES to 81 mPa s of DES containing 6 wt%). However, the differences in the viscosity of water-containing systems were minimal in the current study, and other mechanisms might contribute to the increase in conductivity. Furthermore, when a large amount of water is added, the concentration of ionic species decreases. For example, choline chloride content decreased from 47.21 wt% of CCU12-2 to 31.75 wt% of CCU12-10, yet the conductivity notably increased from 8.23 to 62.36 mS cm−1, respectively.
The conductivity of the systems under study increased along with the increase of choline chloride content. For example, the conductivities of CCU12-10, CCU11-10, and CCU21-10 were 62.36, 77.48, and 85.69 mS cm−1, respectively. Upon increasing the choline chloride content, the number of ionic species is increased, thus resulting in higher conductivity. However, the conductivity is not merely dominated by the amount of choline chloride as RefCC demonstrated the highest choline chloride content (79 wt%) of all water-containing samples, whereas the conductivity was below most of the studied choline chloride–urea systems. For example, CCU12-4 (choline chloride and water content of 42.08 and 21.7 wt%, respectively) demonstrated a conductivity of 28 mS cm−1, which was almost 40% higher compared to RefCC (choline chloride and water content of 79.50 and 20.50 wt%, respectively).
As discussed above, studies have proposed that in a pure state, urea is associated with chloride ions in a choline chloride–urea mixture. The bulky urea group hinders the mobility of chloride ions and thus charge transport, and the low conductivity of CCU12-0 might originate from both, the high viscosity and low mobility of chloride ions. When water is added to the system, some of the urea molecules are replaced by a smaller water molecule45 (molecular mass of urea is 3.33 times higher than that of water and the molecular volume of urea is estimated to be around 2.48 times that of water58), thus making the charge transfer faster. In addition, molecular simulations showed that the replacement of urea by water pulls chloride ions closer together, forming a chain-like structure,45 which could also contribute to the increased conductivity. However, as was shown, with the relatively low conductivity of RefCC, urea also plays a role in the high conductivity of water-containing choline chloride–urea systems, although urea itself exhibits a low conductivity in water (conductivity of RefUrea was 0.64 mS cm−1 at 50 °C). Although the addition of water results in the replacement of urea with water molecules, urea remains part of the nanostructure,45 contributing to the charge transport. However, to better understand the role of urea in the mixture, more research is requested.
Solvent parameters choline chloride–urea mixtures
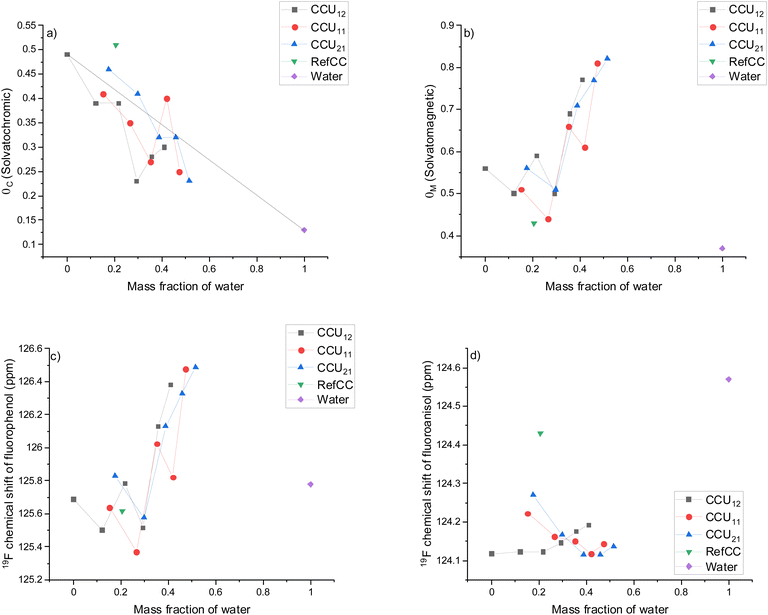
Solvatochromic dyes are widely used to determine solvent parameters,59 such as polarity (ENT), dipolarity/polarizability (π*), and hydrogen bond acceptor (αC) and donor (βC) properties. Alternatively, hydrogen bond acceptor60 and donor39 properties can be determined using solvatomagnetic methods. Here, to investigate the effect of the molar ratio and added water on the solvent properties of choline chloride–urea mixtures, both solvatochromic and solvatomagnetic methods were used.E NT describes the electron transition between the solvent and solute36 and was measured using Nile Red as a solute, and the values are presented in Fig. 3a as a function of the mass fraction of water. As CCU12-0 was the only mixture without added water that remained liquid at room temperature, the dashed line represents the theoretical values that could be predicted by the rule-of-mixture of two individual solvents (CCU12-0 and water).
In all different choline chloride–urea mole ratios, the addition of water decreased the ENT, thus displaying the increase in solvent polarity as a function of water content. The lower polarity of choline chloride–urea mixtures compared to water is in line with values previously obtained using Nile Red.61 Similarly, the increase in polarity as a function of the water content of choline chloride–urea DES has been observed when using betaine dye as a probe.62 Researchers also observed that all the values of CCU12-0 with added water were below the ideal values (dashed line). The deviation of the polarity from the predicted ideal values can be due to the preferred solubility of Nile red in the aqueous phase, which, however, contradicts the poor solubility of Nile red in pure water. Therefore, the possibility exists that an increase in the polarity is due to the alteration of the nanostructure of the solvent mixture by the addition of water, resulting in the formation of a mixture with higher polarity than their individual compounds.
When comparing different molar ratios between choline chloride and urea, it is notable that the polarity parameter of CCU12-0 with the highest urea content showed the lowest values (i.e., highest polarity). The difference between CCU11-0 and CCU21-0 is small, but generally, the CCU11-0 with higher urea content exhibited lower ENT values. When comparing CCU12-2, CCU11-2, CCU21-2, and RefCC, demonstrating similar water contents (12, 15, 18, 20 wt%, respectively), the polarity decreases in the order of their choline chloride content. This observation might be due to the alteration of the alkyl groups (i.e., methyl and methylene) contents of the solvent system36 by the increase in the choline chloride content.
The Kamlet–Taft dipolarity/polarizability (π*) parameter describes the nonspecific interactions such as dipole–dipole and dipole-induced dipole interactions and the polarizability. The π* parameter followed the same trend with ENT (Fig. 3b), as the polarizability increased as a function of water content, similar to that previously observed with the same probe (DENA).62
The solvatochromic hydrogen bond acidity (αC), that is, the ability of the solvent to act as a hydrogen bond donor, was measured using two probes, DENA (to determine π*) and Nile red. Pyridine-N-oxide was used as a sole probe with the solvatomagnetic method to determinate αM. The α parameter measured using both methods displayed similar behaviour, that is, the value increased as a function of water content (Fig. 3c and d). An almost linear correlation was observed with the solvatomagnetic method, whereas some deviations were observed with the solvatochromic method, likely due to the measurement errors caused by the use of two probes. Thus, both methods seem to be suitable for the determination of hydrogen bond donor properties of an aqueous mixture of choline chloride and urea, although the solvatomagnetic method could be more reliable. The αC of CCU12-0 (0.68) and water (1.11) are slightly lower compared to previous literature values (0.92 and 1.17, respectively),54 but in a similar range.
A small, but notable divergence, in the α values, was found when comparing different choline chloride–urea molar ratios. The highest values are observed with CCU12-0 demonstrating the highest urea concentration. Urea exhibits two hydrogen bond donor groups compared to one in choline chloride, and thus, a higher concentration of urea results in a higher α value.
The hydrogen bond basicity (βC) parameter can be determined using the solvatochromic method by comparing UV absorption spectra of 4-nitroaniline (hydrogen bond donor) and 4-nitro-N,N-diethylaniline (reference for non-specific, that is, other than hydrogen bonding interactions). The βC values of all choline chloride–urea mixtures decreased with the addition of water (Fig. 4a), and the decrease is in line with predicted values by the rule-of-mixture. Only a small difference was found between CCU12-0 and CCU11-0; however, the βC values of CCU21-0 were higher compared with the other choline chloride–urea mixtures. Furthermore, the highest βC value (0.51) was observed with RefCC.
The results of βC values measured using the solvatochromic method appear logical, and the values of water (0.13) and CCU12-0 are similar to those observed previously (0.14 and 0.50, respectively). However, the reliability of the solvatochromic methods for the determination of the βC parameter has been debated, especially in the case of amphiprotic solvents, such as water.60 The main disadvantages of the solvatochromic method are reported to be the stoichiometry of hydrogen bonding of the amine group of nitroaniline (i.e., the amine group can form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hydrogen-bonded complex, as well as the mixture of those two with hydrogen bond acceptor) and the formation of hydrogen bonds with the nitro group. Therefore, the solvatomagnetic method, which is based on the fluorine chemical shift of fluorophenol and fluoroanisole, is introduced as an alternative approach for the analysis of the βC parameter.
1 hydrogen-bonded complex, as well as the mixture of those two with hydrogen bond acceptor) and the formation of hydrogen bonds with the nitro group. Therefore, the solvatomagnetic method, which is based on the fluorine chemical shift of fluorophenol and fluoroanisole, is introduced as an alternative approach for the analysis of the βC parameter.
The βM values obtained using the solvatomagnetic method were notably different compared to those determined using the solvatochromic method. The value of CCU12-0 was in a similar range in both methods, but the water exhibited a different influence on βM. For example, the solvatochromic βC value dropped from 0.49 of CCU12-0 to 0.23 of CCU12-6 (water content of 29 wt%). Conversely, βM values determined using the solvatomagnetic method remained at similar levels (between 0.5 and 0.6) until water content exceeded 29 wt%, after which the values increased sharply and reached the value of 0.77 with CCU12-10 (the highest value of 0.82 was observed with CCU21-10). Next, the βM value of water determined using the solvatomagnetic method was 0.44 (close to the value of 0.37 reported previously), and therefore, researchers observed that the βM value determined using the solvatomagnetic method did not obey the rule-of-mixture (Fig. 4b).
When determining the chemical shift of both fluorophenol and fluoroanisol, βM values are mainly dictated by the value of fluorophenol, as the shape of the βM value curves and chemical shift of fluorophenol as a function of water are similar, that is, a notable increase in chemical shift of fluorophenol is observed after water content exceeded 29 wt% (Fig. 4c and d). However, an interesting phenomenon can be seen in the chemical shift of fluoroanisol. When a small amount of water (<29 wt%) was added to the CCU12-0, the chemical shift of fluoroanisol remained at a similar level compared to CCU12-0 without any additional water. However, when the amount of water was further increased, the chemical shift of fluoroanisol approached the chemical shift of pure water.
Previous studies have indicated heterogeneity in the structure of hydrated DESs. Molecular diffusion studies of water with pulsed field gradient NMR led to the conclusion that water is not homogenously mixed with choline chloride and urea, but separate ‘‘microscopic’’ phases are formed at high water concentrations (highest studied water content of 17.5 wt%).54 Furthermore, the addition of a small amount of water (≤6.48 wt%) led to a slight alteration of the chemical structure of the choline chloride–urea mixture (molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), as water contributed to the hydrogen-bonding network.33 At a water content of ∼50 wt%, DES clusters still exist; however, they are diluted with water. The mixtures with water content above ∼50 wt% can, in turn, be described as an aqueous solution of DES compounds. In addition, molecular dynamics simulations indicated that the two dominating nanostructures existed until around 30 wt% of the water content.45 At a water region from 0 to around 30 wt%, the addition of water changed the relative prevalence of these nanostructures; however, water does not alter their salient structural features. Therefore, it is plausible that the constancy of the chemical shift of fluoranisol and only minor changes in the chemical shift of fluorophenol is due to the prevalence of the nanostructure of DES until the amount of added water exceeded 30 wt%. Although significantly more research is needed, the chemical shift of fluoroanisol as a function of added water can be amongst the first experimental signs that the addition of a small amount of water does not notably alter the salient structure of nanoclusters of choline chloride–urea DES.
2), as water contributed to the hydrogen-bonding network.33 At a water content of ∼50 wt%, DES clusters still exist; however, they are diluted with water. The mixtures with water content above ∼50 wt% can, in turn, be described as an aqueous solution of DES compounds. In addition, molecular dynamics simulations indicated that the two dominating nanostructures existed until around 30 wt% of the water content.45 At a water region from 0 to around 30 wt%, the addition of water changed the relative prevalence of these nanostructures; however, water does not alter their salient structural features. Therefore, it is plausible that the constancy of the chemical shift of fluoranisol and only minor changes in the chemical shift of fluorophenol is due to the prevalence of the nanostructure of DES until the amount of added water exceeded 30 wt%. Although significantly more research is needed, the chemical shift of fluoroanisol as a function of added water can be amongst the first experimental signs that the addition of a small amount of water does not notably alter the salient structure of nanoclusters of choline chloride–urea DES.
The solvatomagnetic βM and chemical shift of fluorophenol at different molar ratios of choline chloride and urea demonstrated similar behavior. The values remained at a similar level until around 30 wt% water content and were significantly increased by further addition of water. However, with CCU11-0 and CCU21-0, the chemical shift of fluoroanisol first decreased until around 40% of the added water and then showed some increase (a small plateau is seen with CCU21-0). This different behaviour in the chemical shift of fluoroanisol might indicate that the DES nanostructure is altered in a different manner at various molar ratios.
Choline chloride–urea mixture demonstrating a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (CCU12-0 in the current study) was earlier noted to exhibit the largest deviation (decrease) in the melting point at a water content of 30 wt%34 (corresponding to the largest population Cl-Cl pairs in aqueous DES structure45). In the case of a non-ideal choline chloride–urea mixture at a molar ratio of 2
2 (CCU12-0 in the current study) was earlier noted to exhibit the largest deviation (decrease) in the melting point at a water content of 30 wt%34 (corresponding to the largest population Cl-Cl pairs in aqueous DES structure45). In the case of a non-ideal choline chloride–urea mixture at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (CCU21-0 in the current study), a mixture with lowest melting point is achieved with slightly higher amount of water (around 40 wt%).34 These previous observations are well in line with the observations of the chemical shift of fluoroanisol, and although causation is currently not clear, our results suggest that water demonstrates different effects on the nanostructure of choline chloride–urea mixture, depending on the initial molar ratio of the two compounds.
1 (CCU21-0 in the current study), a mixture with lowest melting point is achieved with slightly higher amount of water (around 40 wt%).34 These previous observations are well in line with the observations of the chemical shift of fluoroanisol, and although causation is currently not clear, our results suggest that water demonstrates different effects on the nanostructure of choline chloride–urea mixture, depending on the initial molar ratio of the two compounds.
The roles of choline chloride and urea molar ratio and added water in cellulose morphology and swelling
The swelling of cellulose fibers induced by choline chloride–urea mixtures was determined by monitoring the fiber widths, and the values were compared with the dimensions of the original fibers as well as several reference systems. All the choline chloride–urea treated fibers showed larger widths (36.71–37.56 μm) compared to the original fibers (36.03 μm), indicating the swelling of cellulose during the treatments (Table 2). These differences in fiber diameters can be considered statistically significant as the analysis is based on the dimensions of thousands of individual fibers (std < 0.1 μm). The maximum swelling of fibers with DES was found to be relatively small (4%), it has been previously shown that a small increase in the diameter plays an important role in the production of cellulosic nanomaterials with mechanical disintegration.14,15,17 Furthermore, the swelling ratio of cellulose fibers was demonstrated to be in line with the swelling of cellulose fibers after (2,2,6,6-tetramethylpiperidn-1-yl)oxyl-mediated oxidation (swelling from 27.71 to 28.50 μm),63 which is amongst the most well-known pre-treatment methods for the production of cellulose nanomaterials. However, recently notable higher swelling from 29.4 to 44.7 μm was demonstrated for aqueous KOH–urea solution, yet a very long swelling time (24 h) at −10 °C was required.64| Sample | Width (μm) | Length (mm) | Curl (%) | Kink (m−1) | Kink angle (°) | Fines (%) |
|---|---|---|---|---|---|---|
| a No mixing due to the high viscosity. | ||||||
| Original pulp | 36.03 | 1.839 | 15.23 | 4065 | 31.43 | 10.44 |
| CCU12-0 | 36.81 | 1.818 | 15.49 | 4060 | 31.09 | 9.77 |
| CCU12-2 | 37.05 | 1.884 | 15.77 | 4015 | 30.91 | 8.05 |
| CCU12-4 | 37.22 | 1.848 | 17.14 | 4194 | 32.16 | 8.21 |
| CCU12-6 | 36.62 | 1.831 | 16.05 | 4086 | 31.31 | 8.17 |
| CCU12-8 | 36.77 | 1.832 | 16.62 | 4116 | 31.64 | 7.34 |
| CCU12-10 | 36.99 | 1.865 | 16.26 | 3999 | 31.39 | 7.87 |
| CCU11-0 | 37.20 | 1.846 | 16.33 | 4193 | 31.63 | 9.49 |
| CCU11-2 | 37.10 | 1.87 | 16.36 | 4082 | 31.96 | 7.21 |
| CCU11-4 | 36.94 | 1.856 | 16.16 | 4115 | 31.84 | 7.88 |
| CCU11-6 | 36.85 | 1.87 | 16.93 | 4277 | 32.49 | 7.04 |
| CCU11-8 | 37.09 | 1.826 | 17.38 | 4271 | 33.34 | 8.62 |
| CCU11-10 | 36.88 | 1.865 | 16.32 | 4115 | 31.74 | 7.36 |
| CCU21-0a | 37.56 | 1.858 | 16.39 | 3916 | 32.55 | 7.48 |
| CCU21-2 | 37.12 | 1.877 | 15.85 | 4053 | 31.53 | 7.65 |
| CCU21-4 | 37.09 | 1.847 | 16.71 | 4100 | 32.77 | 8.37 |
| CCU21-6 | 36.71 | 1.843 | 15.79 | 3940 | 31.33 | 8.02 |
| CCU21-8 | 36.9 | 1.848 | 15.61 | 3854 | 31.57 | 8.12 |
| CCU21-10 | 37.12 | 1.851 | 17.36 | 4154 | 32.62 | 7.67 |
| RefCC | 37.04 | 1.865 | 15.92 | 4043 | 31.62 | 7.09 |
| RefUrea | 36.98 | 1.851 | 16.01 | 3884 | 30.61 | 7.84 |
| RefWater | 37.00 | 1.795 | 18.34 | 4398 | 32.83 | 8.63 |
| RefWater (pH 10) | 36.70 | 1.766 | 19.02 | 4504 | 34.78 | 9.26 |
| RefCC (pH 10) | 36.98 | 1.857 | 16.24 | 4082 | 31.99 | 7.42 |
Interestingly, the deep eutectic point composition, that is CCU12-0, resulted in the lowest width value of all the samples without the added water, and the width of the treated fibers increased as a function of the amount of choline chloride, with the CCU21-0 treated sample showing the largest width. This behavior might be attributed to the increased chlorine ion content of the solvent systems.65 Based on the molecular simulations, the main interaction between DES of choline chloride and urea with cellulose is via hydrogen bonding of the chloride ion of choline chloride and hydroxyl groups of cellulose.65 Therefore, the increase of choline chloride in the mixture could increase the interaction probability between cellulose and chloride ions and, thus, result in a higher degree of swelling. However, the physical properties of the solvents might also contribute to the swelling, and in the following section, the effect of the mixing on the swelling is discussed.
It is notable that when water was added, all the choline chloride–urea mixtures showed a similar degree of swelling of cellulose fibers. In addition, the reference systems showed fiber swelling that was comparable with those of choline chloride–urea systems with added water and even with those of CCU12-0 and CCU11-0.
Also, the choline chloride–urea mixtures slightly affected the length of the fibers. However, the overall level of the fiber length was similar, indicating that no cutting or major degradation of fibers was observed, and also supported the small changes in the fine content of the samples. Only notable differences in the fiber length were observed when cellulose fibers were treated with pure water or water at a pH of 10 as both samples showed fiber lengths lower than 1.8 mm. The change in the fiber length by the pure and alkaline water treatment might be the deformation of the fiber, as the percentage of the curls and kinks were notably higher in these samples compared to others. Therefore, it is apparent that although pure water can result in fiber swelling, DES treatments might be more desirable if fiber deformation needs are to be minimized. The difference in the fiber deformation plausibly originates from different viscosities of the systems. In highly viscous systems (choline chloride–urea mixtures), more mechanical energy is consumed by the solvent, and a lower amount of energy is exposed to fibers; thus, the deformation induced to fibers is not as severe as in the case of low-viscosity solutions (water). However, several other factors, such as the loosening of the fiber structure by the solvent-induced swelling can be expected to demonstrate an effect on the formation of curl and kinks, and more research is needed to verify the hypothesis related to the effect of viscosity.
Influence of mixing on the fiber swelling in choline chloride–urea mixtures
The CCU21-0 was a highly viscous solution, and the mixing of pulp suspension was not possible with a magnetic stirrer. Therefore, the role of mixing was also elucidated with all other choline chloride–urea systems without the added water and with the highest water content as well as with pure water (Table 3). In all treatments, the width of the fibers was higher when no stirring was applied. Interestingly, without stirring, the highest swelling of the fibers was observed in the case of CCU12-10 (fiber width of 38.11 μm), demonstrating a water content of 41 wt%. Therefore, the aqueous choline chloride–urea system can increase fiber swelling, particularly in the absence of stirring.| Sample | Width (μm) | Length (mm) | Curl (%) | Kink (m−1) | Kink angle (°) | Fines (%) |
|---|---|---|---|---|---|---|
| CCU12-0 | 37.9 | 1.802 | 19.93 | 4483 | 35.04 | 8.91 |
| CCU12-10 | 38.11 | 1.784 | 21.1 | 4730 | 36.42 | 9.38 |
| CCU11-0 | 37.94 | 1.831 | 19.47 | 4475 | 34.94 | 8.32 |
| CCU11-20 | 38.09 | 1.791 | 20.7 | 4698 | 36.07 | 10.67 |
| CCU21-0 | 37.71 | 1.827 | 18.43 | 4421 | 34.15 | 7.68 |
| CCU21-10 | 37.74 | 1.773 | 20.95 | 4777 | 36.68 | 10.6 |
| RefWater | 37.86 | 1.763 | 21.13 | 4880 | 36.94 | 10.05 |
Mechanical treatments, such as mixing, can cause changes in the ultrastructure of the fibers with the closure of micropores and opening of macropores.66 The closure of the micropores could in turn decrease the interaction of the solvent and cellulose fibers, which in turn leads to a lower degree of swelling, as the solvent is unable to penetrate inside of the fibers as efficiently during the mixing. Also, it is plausible that the mixing changes the orientation of the nanostructure of molecular clusters of the choline chloride–urea complex (as demonstrated by the non-Newtonian viscosity of mixtures), which can then alter their interaction with cellulose fibers (solid surface).
Conclusions
In this study, the properties of choline chloride–urea mixtures demonstrating variable molar ratios were investigated as a function of added water. A clear effect of the added water on the conductivity (increase) and pH (decrease) of the mixtures was observed. These changes are not merely due to the dilution of the solution by the addition of water but are related to the chemical composition of the mixtures, that is, the presence of choline chloride and urea and their molar ratios. Next, the participation of water in the formation of the molecular clusters with choline chloride and urea was indicated by the cone-plate viscosity analysis as the addition of water did not decrease the viscosity values nor changed the viscosity behavior of the mixtures from non-Newtonian to Newtonian, even at the highest water content (around 50 wt%). Solvent parameters, that is, polarity, dipolarity/polarizability, and hydrogen bond acceptor property, determined using the solvatochromic method showed small, but notable, deviations from the theoretical values predicted by the rule-of-mixture. Hydrogen bond acceptor values, calculated using solvatomagnetic methods, were in line with those obtained using solvatochromic methods, indicating that both methods are suitable for the determination of hydrogen bond acidity of aqueous choline chloride–urea mixtures. However, hydrogen bond basicity determined using solvatochromic and magnetic methods exhibited almost opposite trends. Based on the results obtained here, whether the differences between the two methods are due to the biased results originating from the complex solvent system or if these methods measure different behaviors cannot be concluded. Thus, solvent parameters of the binary or ternary protic systems should be critically evaluated, especially when compared with those of molecular solvents. Inspection of the chemical shift of the solvatomagnetic probes, especially those of fluoroanisol in choline chloride: urea molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, indicated that the chemical environment remained similar until the addition of around 30 wt% of water, which is in agreement with previous molecular simulations about the molecular clusters of DES. Furthermore, although no direct connection between the swelling of cellulose and solvent parameters could be made based on current data, it was demonstrated that an aqueous mixture can efficiently increase the diameters of cellulose fibers in the absence of mixing, and the best swelling capacity was achieved with the choline chloride–urea–water molar ratio of 1
2, indicated that the chemical environment remained similar until the addition of around 30 wt% of water, which is in agreement with previous molecular simulations about the molecular clusters of DES. Furthermore, although no direct connection between the swelling of cellulose and solvent parameters could be made based on current data, it was demonstrated that an aqueous mixture can efficiently increase the diameters of cellulose fibers in the absence of mixing, and the best swelling capacity was achieved with the choline chloride–urea–water molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (water content of 41 wt%.). An aqueous solvent system could significantly enhance for example the pre-treatment of cellulose fibers prior to enzymatic hydrolysis or nanofibrillation as energy-intensive drying of the solvent and fibers could be avoided.
10 (water content of 41 wt%.). An aqueous solvent system could significantly enhance for example the pre-treatment of cellulose fibers prior to enzymatic hydrolysis or nanofibrillation as energy-intensive drying of the solvent and fibers could be avoided.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was conducted as a part of the Value for Cellulosics (ValCel) project (No. 42679/31/2020) funded by the ExpandFibre program of Business Finland. Part of the work was carried out with the support of the Centre for Materials Analysis, University of Oulu. We acknowledge Jani Österlund for his assistance in the experiments.Notes and references
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- M. A. R. Martins, S. P. Pinho and J. A. P. Coutinho, J. Solut. Chem., 2019, 48, 962–982 CrossRef CAS.
- S. J. Bryant, A. J. Christofferson, T. L. Greaves, C. F. McConville, G. Bryant and A. Elbourne, J. Colloid Interface Sci., 2022, 608, 2430–2454 CrossRef CAS PubMed.
- M. Hayyan, M. A. Hashim, A. Hayyan, M. A. Al-Saadi, I. M. AlNashef, M. E. S. Mirghani and O. K. Saheed, Chemosphere, 2013, 90, 2193–2195 CrossRef CAS PubMed.
- D. Lapeña, D. Errazquin, L. Lomba, C. Lafuente and B. Giner, Environ. Sci. Pollut. Res., 2021, 28, 8812–8821 CrossRef PubMed.
- Q. Wen, J.-X. Chen, Y.-L. Tang, J. Wang and Z. Yang, Chemosphere, 2015, 132, 63–69 CrossRef CAS PubMed.
- A. Abo-Hamad, M. Hayyan, M. A. AlSaadi and M. A. Hashim, Chem. Eng. J., 2015, 273, 551–567 CrossRef CAS.
- M. L. Gars, L. Douard, N. Belgacem and J. Bras, Cellulose Nanocrystals: From Classical Hydrolysis to the Use of Deep Eutectic Solvents, IntechOpen, 2019 Search PubMed.
- M. Zdanowicz, K. Wilpiszewska and T. Spychaj, Carbohydr. Polym., 2018, 200, 361–380 CrossRef CAS PubMed.
- J. A. Sirviö, J. Mater. Chem. A, 2019, 7, 755–763 RSC.
- M. A. Smirnov, M. P. Sokolova, D. A. Tolmachev, V. K. Vorobiov, I. A. Kasatkin, N. N. Smirnov, A. V. Klaving, N. V. Bobrova, N. V. Lukasheva and A. V. Yakimansky, Cellulose, 2020, 27, 4305–4317 CrossRef CAS.
- S. H. Osong, S. Norgren and P. Engstrand, Cellulose, 2016, 23, 93–123 CrossRef CAS.
- J. A. Sirviö, M. Visanko and H. Liimatainen, Green Chem., 2015, 17, 3401–3406 RSC.
- J. A. Sirviö, K. Hyypiö, S. Asaadi, K. Junka and H. Liimatainen, Green Chem., 2020, 22, 1763–1775 RSC.
- P. Li, J. A. Sirviö, A. Haapala and H. Liimatainen, ACS Appl. Mater. Interfaces, 2017, 9, 2846–2855 CrossRef CAS PubMed.
- A. Hosseinmardi, P. K. Annamalai, L. Wang, D. Martin and N. Amiralian, Nanoscale, 2017, 9, 9510–9519 RSC.
- T. Suopajärvi, J. A. Sirviö and H. Liimatainen, Carbohydr. Polym., 2017, 169, 167–175 CrossRef PubMed.
- P. Li, J. A. Sirviö, B. Asante and H. Liimatainen, Carbohydr. Polym., 2018, 199, 219–227 CrossRef CAS PubMed.
- J. A. Sirviö, Carbohydr. Polym., 2018, 198, 34–40 CrossRef PubMed.
- E. E. Jaekel, J. A. Sirviö, M. Antonietti and S. Filonenko, Green Chem., 2021, 23, 2317–2323 RSC.
- J. A. Sirviö and M. Visanko, Biomacromolecules, 2019, 20, 2413–2420 CrossRef PubMed.
- J. A. Sirviö and M. Visanko, J. Mater. Chem. A, 2017, 5, 21828–21835 RSC.
- J. A. Sirviö and J. P. Heiskanen, ChemSusChem, 2017, 10, 455–460 CrossRef PubMed.
- X. Meng, K. Ballerat-Busserolles, P. Husson and J.-M. Andanson, New J. Chem., 2016, 40, 4492–4499 RSC.
- N. Guajardo, P. Domínguez de María, K. Ahumada, R. A. Schrebler, R. Ramírez-Tagle, F. A. Crespo and C. Carlesi, ChemCatChem, 2017, 9, 1393–1396 CrossRef CAS.
- Y. Marcus, ACS Sustainable Chem. Eng., 2017, 5, 11780–11787 CrossRef CAS.
- M. M. Nolasco, S. N. Pedro, C. Vilela, P. D. Vaz, P. Ribeiro-Claro, S. Rudić, S. F. Parker, C. S. R. Freire, M. G. Freire and A. J. D. Silvestre, Front. Phys, 2022, 10, 834571 CrossRef.
- E. S. Morais, P. V. Mendonça, J. F. J. Coelho, M. G. Freire, C. S. R. Freire, J. A. P. Coutinho and A. J. D. Silvestre, ChemSusChem, 2018, 11, 753–762 CrossRef CAS PubMed.
- M. E. Di Pietro, M. Tortora, C. Bottari, G. Colombo Dugoni, R. V. Pivato, B. Rossi, M. Paolantoni and A. Mele, ACS Sustainable Chem. Eng., 2021, 9, 12262–12273 CrossRef CAS.
- Y. Xie, H. Dong, S. Zhang, X. Lu and X. Ji, J. Chem. Eng. Data, 2014, 59, 3344–3352 CrossRef CAS.
- Q. Gao, Y. Zhu, X. Ji, W. Zhu, L. Lu and X. Lu, Fluid Phase Equilib., 2018, 470, 134–139 CrossRef CAS.
- M. Bučko, S. Roy, P. Valverde-Armas, A. Onjia, A. C. Bastos and J. B. Bajat, J. Electrochem. Soc., 2018, 165, H1059 CrossRef.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Angew. Chem., Int. Ed., 2017, 56, 9782–9785 CrossRef CAS PubMed.
- P. J. Smith, C. B. Arroyo, F. Lopez Hernandez and J. C. Goeltz, J. Phys. Chem. B, 2019, 123, 5302–5306 CrossRef CAS PubMed.
- M. Gilmore, M. Swadzba-Kwasny and J. D. Holbrey, J. Chem. Eng. Data, 2019, 64, 5248–5255 CrossRef CAS.
- A. K. Dwamena and D. E. Raynie, J. Chem. Eng. Data, 2020, 65, 640–646 CrossRef CAS.
- A. R. R. Teles, E. V. Capela, R. S. Carmo, J. A. P. Coutinho, A. J. D. Silvestre and M. G. Freire, Fluid Phase Equilib., 2017, 448, 15–21 CrossRef CAS PubMed.
- Q. Xia, Y. Liu, J. Meng, W. Cheng, W. Chen, S. Liu, Y. Liu, J. Li and H. Yu, Green Chem., 2018, 20, 2711–2721 RSC.
- H. Schneider, Y. Badrieh, Y. Migron and Y. Marcus, Z. Phys. Chem., 1992, 177, 143–156 CrossRef CAS.
- C. Laurence, S. Mansour, D. Vuluga and J. Legros, Green Chem., 2021, 23, 1816–1822 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- Y. Elhamarnah, H. Qiblawey, M. S. Nasser and A. Benamor, Sci. Total Environ., 2020, 708, 134848 CrossRef CAS PubMed.
- G. L. Burrell, N. F. Dunlop and F. Separovic, Soft Matter, 2010, 6, 2080–2086 RSC.
- O. S. Hammond, H. Li, C. Westermann, A. Y. M. Al-Murshedi, F. Endres, A. P. Abbott, G. G. Warr, K. J. Edler and R. Atkin, Nanoscale Horiz., 2018, 4, 158–168 RSC.
- L. Sapir and D. Harries, J. Chem. Theory Comput., 2020, 16, 3335–3342 CrossRef CAS PubMed.
- A. Yadav and S. Pandey, J. Chem. Eng. Data, 2014, 59, 2221–2229 CrossRef CAS.
- G. E. Brodale and W. F. Giauque, J. Phys. Chem., 1962, 66, 2051 CrossRef CAS.
- R. N. Havemeyer, J. Pharm. Sci., 1966, 55, 851–853 CrossRef CAS PubMed.
- A. Triolo, F. Lo Celso, M. Brehm, V. Di Lisio and O. Russina, J. Mol. Liq., 2021, 331, 115750 CrossRef CAS.
- K. M. Harmon, A. C. Akin, G. F. Avci, L. S. Nowos and M. B. Tierney, J. Mol. Struct., 1991, 244, 223–236 CrossRef CAS.
- A. T. Celebi, N. Dawass, O. A. Moultos and T. J. H. Vlugt, J. Chem. Phys., 2021, 154, 184502 CrossRef CAS PubMed.
- F. Wang, S. Ma, D. Zhang and R. G. Cooks, J. Phys. Chem. A, 1998, 102, 2988–2994 CrossRef CAS.
- J. P. Chen and K. Isa, J. Mass Spectrom. Soc. Jpn., 1998, 46, 299–303 CrossRef CAS.
- C. D’Agostino, L. F. Gladden, M. D. Mantle, A. P. Abbott, I. A. Essa, A. Y. M. Al-Murshedi and R. C. Harris, Phys. Chem. Chem. Phys., 2015, 17, 15297–15304 RSC.
- H. Sun, Y. Li, X. Wu and G. Li, J. Mol. Model., 2013, 19, 2433–2441 CrossRef CAS PubMed.
- E. Iwamoto, J. Nishimoto, T. Yokoyama, K. Yamamoto and T. Kumamaru, J. Chem. Soc., Faraday Trans., 1991, 87, 1537–1543 RSC.
- C. Du, B. Zhao, X.-B. Chen, N. Birbilis and H. Yang, Sci. Rep., 2016, 6, 1–14 CrossRef PubMed.
- H. Kokubo and B. M. Pettitt, J. Phys. Chem. B, 2007, 111, 5233–5242 CrossRef CAS PubMed.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- C. Laurence, J. Legros, P. Nicolet, D. Vuluga, A. Chantzis and D. Jacquemin, J. Phys. Chem. B, 2014, 118, 7594–7608 CrossRef CAS PubMed.
- M. Q. Farooq, N. M. Abbasi and J. L. Anderson, J. Chromatogr. A, 2020, 1633, 461613 CrossRef CAS PubMed.
- A. Pandey and S. Pandey, J. Phys. Chem. B, 2014, 118, 14652–14661 CrossRef CAS PubMed.
- J. Levanič, V. P. Šenk, P. Nadrah, I. Poljanšek, P. Oven and A. Haapala, ACS Sustainable Chem. Eng., 2020, 8, 17752–17762 CrossRef.
- C. Wang, L. Luo, W. Zhang, S. Geng, A. Wang, Z. Fang and Y. Wen, Cellulose, 2022, 29, 8623–8636 CrossRef CAS.
- V. Alizadeh and B. Kirchner, J. Chem. Phys., 2021, 155, 084501 CrossRef CAS PubMed.
- O. Joutsimo, Effect of mechanical treatment on softwood kraft fiber properties, Helsinki University of Technology, 2004 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Structure of the chemicals used; viscosity of RefCC at room temperature and CCU12-0 and CCU11-0 at 100 °C. See DOI: https://doi.org/10.1039/d2cp04119g |
| This journal is © the Owner Societies 2022 |