Non-covalent bonds in group 1 and group 2 elements: the ‘alkalene bond’†
Abstract
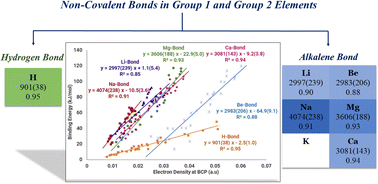
The non-covalent bonds formed by group 1 and group 2 elements were systematically analysed by ab initio calculations at the MP2/aug-cc-pVDZ (for Ca, 6-311++G(2df,p) basis sets were used) level of theory to classify the weak bonds, followed by Atoms in Molecules (AIM) analysis of the ab initio wave functions. It has been established that there is a strong correlation between the electron density at the non-covalent bond critical point (BCP) and the binding energy for each homogeneous sample of complexes. The slopes of the electron density versus binding energy plot have been obtained for group 1 and group 2 donor molecules (Dn–X⋯A, for X = H, D = F/–OH/–SH, for X = Li, Na, D = F/Cl/Br and for X = Be, Mg, and Ca, D = F/Cl/H) with a set of acceptor molecules (A), which includes H2O, NH3, H2S, PH3, HCHO, C2H4, HCN, CO, CH3OH and CH3OCH3. The bonds formed by group 1 (except H-bonds) and group 2 belong to a high slope dominated by electrostatics, with several similarities, leading us to propose a common name, ‘alkalene bond’, for non-covalent bonding in alkali and alkaline earth metals.


 Please wait while we load your content...
Please wait while we load your content...