 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Radiation effects, zero thermal expansion, and pressure-induced phase transition in CsMnCo(CN)6†
Hanna L. B.
Boström
 *a,
Andrew B.
Cairns
*a,
Andrew B.
Cairns
 bc,
Muzi
Chen
bc,
Dominik
Daisenberger
d,
Christopher J.
Ridley
bc,
Muzi
Chen
bc,
Dominik
Daisenberger
d,
Christopher J.
Ridley
 e and
Nicholas P.
Funnell
e and
Nicholas P.
Funnell
 e
e
aMax Planck Institute for Solid State Research, Heisenbergstraße 1, D-70569, Stuttgart, Germany. E-mail: h.bostroem@fkf.mpg.de
bDepartment of Materials, Imperial College London, Royal School of Mines, Exhibition Road, SW7 2AZ, London, UK
cLondon Centre for Nanotechnology, Imperial College London, SW7 2AZ, London, UK
dDiamond Light Source Ltd., Harwell Campus, Didcot, OX11 0DE, UK
eISIS Neutron and Muon Source, Rutherford Appleton Laboratory, Harwell Campus, Didcot, OX11 0QX, UK
First published on 6th October 2022
Abstract
The Prussian blue analogue CsMnCo(CN)6 is studied using powder X-ray and neutron diffraction under variable temperature, pressure, and X-ray exposure. It retains cubic F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m symmetry in the range 85–500 K with minimal thermal expansion, whereas a phase transition to P
3m symmetry in the range 85–500 K with minimal thermal expansion, whereas a phase transition to P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) n2 occurs at ∼2 GPa, driven by octahedral tilting. A small lattice contraction occurs upon increased X-ray dose. Comparisons with related systems indicate that the CsI ions decrease the thermal expansion and suppress the likelihood of phase transformations. The results improve the understanding of the stimuli-responsive behaviour of coordination polymers.
n2 occurs at ∼2 GPa, driven by octahedral tilting. A small lattice contraction occurs upon increased X-ray dose. Comparisons with related systems indicate that the CsI ions decrease the thermal expansion and suppress the likelihood of phase transformations. The results improve the understanding of the stimuli-responsive behaviour of coordination polymers.
Understanding how external perturbations affect a material, in particular strain effects and the propensity for phase transitions, is a key research area in solid-state chemistry. Apart from the fundamental interest in stimuli-responsive behaviour, this is also critical to many applications. By way of example, a high resistance towards phase transitions is desirable for electrode materials during cation intercalation1,2 or for pharmaceuticals during compression into tablets.3 Conversely, low mechanical stability allows for glass processing by pressure-induced amorphisation.4,5 Moreover, the response to e.g. temperature or pressure fluctuations is an important consideration for materials exposed to changing conditions during operation or processing.6
Mechanical properties in framework materials are often linked to the amount of void space. For example, the thermal expansion and bulk modulus of the archetypal metal–organic framework UiO-66 can be tuned by introducing missing-cluster defects (vacancies),7,8 whereas interstitial KI ions in KMn[Ag(CN)2]3 increase the pressure range—but decrease the magnitude—of the negative linear compressibility.9 A family known for their large stoichiometric diversity—and thus variable void space—is the Prussian blue analogues (PBAs),10,11 which exhibit versatile properties on account of the high tunability.12–14 PBAs have the general formula AxM[M′(CN)6]y·nH2O (A = alkali metal and M/M′ = transition metals) and can be described as cyanide analogues of double perovskites. The behaviour of alkali-containing PBAs resembles that of oxides, in the sense that magnetic order and cation intercalation are possible.12,13
Here we undertake an in-depth study of the stimuli-responsive behaviour of the PBA CsMnCo(CN)6, using powder X-ray and neutron diffraction (XRD/ND) under variable temperature, pressure, and X-ray exposure. CsMnCo(CN)6 resides at the dense end of the PBA stoichiometry spectrum and thus provides an insight into the limiting mechanical behaviour obtainable by inclusion of large cations. The CsI ions reduce the free space available for compression and transverse vibrations, and can thus be expected to enhance the rigidity. Many PBAs are explored as sorbents for radioactive CsI ions from nuclear waste,15,16 which further motivates the study of Cs-containing PBAs. We find that CsMnCo(CN)6 transitions to a tetragonal phase at 2 GPa, driven by octahedral tilting, but features no thermal phase transitions and a very low thermal expansion. Like certain other metal–cyanides, CsMnCo(CN)6 is susceptible to X-ray radiation and contracts slightly as the exposure increases. The results are discussed in the context of other PBAs.
Polycrystalline CsMnCo(CN)6 was prepared by direct combination of aqueous solutions of an appropriate MnII salt and K3Co(CN)6 in an excess of CsCl [see ESI† for details]. The large radius of Cs should prevent water from being included and hydration—which is integral to many other PBAs17–19—is not expected to play a major role here. Powder X-ray diffraction (XRD) confirms that CsMnCo(CN)6 adopts the non-centrosymmetric space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m at ambient conditions [Fig. 1(a)]. This corresponds to the typical double perovskite structure with a rocksalt arrangement of MnII and CoIII ions linearly joined by cyanide ions. Each unit cell contains eight interstitial A-sites, half of which are occupied by CsI ions in an alternating arrangement, leading to the non-centrosymmetric symmetry. However, this order is not complete, as the lesser-occupied sites still show an occupancy of ca. 0.2 CsI ions, which leads to substantial broadening of reflections where h + k + l = 2n + 1 [Fig. S1, ESI†].
3m at ambient conditions [Fig. 1(a)]. This corresponds to the typical double perovskite structure with a rocksalt arrangement of MnII and CoIII ions linearly joined by cyanide ions. Each unit cell contains eight interstitial A-sites, half of which are occupied by CsI ions in an alternating arrangement, leading to the non-centrosymmetric symmetry. However, this order is not complete, as the lesser-occupied sites still show an occupancy of ca. 0.2 CsI ions, which leads to substantial broadening of reflections where h + k + l = 2n + 1 [Fig. S1, ESI†].
Exposure to X-ray radiation can affect metal–cyanide frameworks, manifesting as changes in the lattice constants.20–22 To investigate whether this occurs in CsMnCo(CN)6, ten consecutive XRD patterns were collected on the same part of the sample at beamline I11, Diamond Light Source. This reveals a small, but significant and continuous, decrease in the lattice parameter as a function of increased radiation dose [Fig. S2–S4 and Table S2, ESI†], accompanied by a very slight decrease in the diffraction intensity. Radiation-induced unit cell contractions were recently named “negative X-ray expansion” (NXE),22 as this contrasts with the lattice expansion commonly seen as a sign of radiation damage in macromolecular and small-molecule crystallography.23–25 As the dose was not monitored, the magnitude of NXE cannot be quantified, yet CsMnCo(CN)6 belongs to the group of NXE materials—a group that is likely to grow as more systems are explored.
CsMnCo(CN)6 was studied by variable-pressure X-ray diffraction up to ∼8 GPa and neutron diffraction up to ∼5 GPa. While reflections broaden slightly, the X-ray crystallinity is maintained within this pressure range [Fig. 2]. The ND patterns suffer more severe peak broadening upon increasing pressure, which is attributed to differences in the experimental set-up. As previously noted in an X-ray study,26 CsMnCo(CN)6 transitions to tetragonal symmetry around 2 GPa, with cell parameters related to that of the cubic parent (ac) by 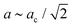 and c ∼ ac. This high-pressure phase could be fitted satisfactorily with either I
and c ∼ ac. This high-pressure phase could be fitted satisfactorily with either I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) or P
or P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) n2 symmetry, with a slight preference for the latter. Both space groups result from octahedral tilting,27 but with different periodicity: I
n2 symmetry, with a slight preference for the latter. Both space groups result from octahedral tilting,27 but with different periodicity: I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) implies out-of-phase tilts (a0a0b− in Glazer notation28) and P
implies out-of-phase tilts (a0a0b− in Glazer notation28) and P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) n2 from in-phase tilts (a0a0b+).27 As these structures solely differ in the positions of the cyanide ions, they are not easily distinguished based on X-ray data alone. However, by refining against both X-ray and neutron data at 3.7 GPa, we here confirm that the correct space group is P
n2 from in-phase tilts (a0a0b+).27 As these structures solely differ in the positions of the cyanide ions, they are not easily distinguished based on X-ray data alone. However, by refining against both X-ray and neutron data at 3.7 GPa, we here confirm that the correct space group is P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) n2 [Fig. S5 and Table S3, ESI†]. The in-phase a0a0b+ octahedral tilt is relatively common in PBAs with large A-site cations29 and is also prevalent in halide perovskites,30 but rarely occurs in oxides.31
n2 [Fig. S5 and Table S3, ESI†]. The in-phase a0a0b+ octahedral tilt is relatively common in PBAs with large A-site cations29 and is also prevalent in halide perovskites,30 but rarely occurs in oxides.31
The tetragonal phase persists across the pressure range studied here, with anisotropic and pressure-dependent compressibilities (Ki) [Fig. 2 and Fig. S6, Table S4, ESI†]. Initially, the a axis is softer than the c axis by an order of magnitude, with Ka = 21.1(5) TPa−1 and Kc = 1.8(3) TPa−1. These values are considerably lower than the compressibilites of the tetragonal high-pressure phase of RbMnCo(CN)6 (Ka = 36(2) TPa−1, Kc = 5.3(6) TPa−1),26 which shows that the CsI ions decrease the flexibility of the framework. The large difference between Ka and Kc indicates that the compression mechanism after the phase transition mainly involves increasing tilting magnitude, which contracts the ab plane. As the pressure is raised, the a axis becomes increasingly stiff and the c axis softens slightly, such that Ka ∼ Kc above ∼6.5 GPa. Using a third-order Murnaghan equation of state to fit the volume–pressure data, the bulk modulus (B0) is 4.4(4) GPa with a first pressure derivative  of 7.08(8) [Fig. S7, ESI†]; hence, the high-pressure phase of CsMnCo(CN)6 is initially very compressible, yet hardens rapidly as pressure increases.
of 7.08(8) [Fig. S7, ESI†]; hence, the high-pressure phase of CsMnCo(CN)6 is initially very compressible, yet hardens rapidly as pressure increases.
As a result of the changes to the compressibility, the tetragonality  —effectively a measure of the tilting amplitude—initially increases from unity and subsequently reaches a plateau at a value of ∼1.06 at higher pressures [Fig. 2]. This suggests that the contribution of the static tilting to the overall compression is reduced, and could potentially indicate the presence of an additional low-energy dynamic tilt mode affecting the c axis. The analogous RbMnCo(CN)6 shows the same F
—effectively a measure of the tilting amplitude—initially increases from unity and subsequently reaches a plateau at a value of ∼1.06 at higher pressures [Fig. 2]. This suggests that the contribution of the static tilting to the overall compression is reduced, and could potentially indicate the presence of an additional low-energy dynamic tilt mode affecting the c axis. The analogous RbMnCo(CN)6 shows the same F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m → P
3m → P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) n2 transition as the Cs compound, but has a further transition to the polar space group Pn, driven by a tilt along c (a−a−b+).26 This tilt mode perhaps appears locally at CsMnCo(CN)6 at these pressures, although no global, long-range phase transition was seen.
n2 transition as the Cs compound, but has a further transition to the polar space group Pn, driven by a tilt along c (a−a−b+).26 This tilt mode perhaps appears locally at CsMnCo(CN)6 at these pressures, although no global, long-range phase transition was seen.
Variable-temperature X-ray diffraction was used to study CsMnCo(CN)6 in the range 500–85 K upon cooling. To avoid the aforementioned X-ray effects, every XRD pattern was collected from a previously non-irradiated part of the sample. Due to the finite length of the capillary, this approach necessitated a change of capillary—with material from the same sample batch—between 300 and 260 K. CsMnCo(CN)6 retains its cubic structure in the entire temperature range without noticeable changes to the XRD patterns [Fig. S8 and S9, ESI†].
Remarkably, the lattice parameters remain nearly unchanged [Fig. 3(a) and Table S5, ESI†] as a function of temperature, with the discontinuity between 300 and 260 K resulting from the capillary change. Given the extremely low overall expansivity, slight differences between different capillaries give changes in lattice parameter on the same scale as the expansion. The linear and volumetric coefficients of thermal expansion are 0.46(7) MK−1 and 2.3(6) MK−1, respectively. These values are very low and place CsMnCo(CN)6 amongst the zero thermal expansion (ZTE) materials. Similarly low expansivities are also found for the closely related CsMnFe(CN)6 and other Cs-containing PBAs.32,33 The trend that Cs decreases the expansivitiy relative to Rb also appears to generally hold for thermal expansion, albeit the effect is smaller.33 ZTE is of interest for many applications where the material is subjected to large temperature fluctuations, ranging from e.g. cookware to high-precision optics.34
For a given application, both the range and magnitude of the thermal expansion are important considerations.35 Thus for context, the linear thermal expansion coefficients of numerous cubic PBAs are given in Fig. 3(b) as a function of the corresponding temperature range. An enlarged view is provided in Fig. S10 (ESI†). The entries are coloured according to stoichiometry: recalling the general formula of a PBA as AxM[M′(CN)6]y·nH2O, alkali-metal-containing compounds (teal) are defined as x > 0, stoichiometric (purple) as x = y = 0, and defective (grey) as y > 0.3. While this provides a useful categorisation for discussion of stoichiometrical effects on the thermal expansion behaviour, it should be remembered that the stoichiometry of PBAs is continuously variable, rather than discrete.36
Stoichiometry clearly plays a role in dictating the thermal expansion of PBAs [Fig. 3(b)]. The defective PBAs show the largest spread of expansivities, and span large negative and positive values, depending on the framework metal ions.37 This bears testament to the high structural flexibility resulting from the missing M′(CN)6 clusters. However, this flexibility is also affected by guest species, typically water;38 yet there are few studies on the interplay of hydration and thermal expansion in these materials. Stoichiometric and alkali metal-containing PBAs typically show thermal expansion coefficients closer to zero (|αa| < 15 MK−1), and many compounds in these two categories show ZTE. The introduction of alkali metals—or guest molecules—often leads to ZTE by inhibiting the low-energy transverse phonons responsible for NTE,39,40 but this is evidently not a necessary condition. Several studies have shown a relationship between the lattice parameter and the thermal expansion in PBAs,33,40,41 and so an appropriate choice of framework metal ions may also drive ZTE, e.g. FeCo(CN)6.42 Taken together, vacancies exert a large influence on the thermal response of PBAs, and compositional factors play a key role for stoichiometric and alkali-metal-containing compounds. Similar effects have also been noted for the bulk moduli.26
To conclude, the Prussian blue analogue CsMnCo(CN)6 generally shows a low responsiveness to external temperature and pressure variation. This manifests as a lower propensity for pressure-induced phase transitions than the isostructural RbMnCo(CN)6, and near-zero thermal expansion. We attribute this to the stabilising role of the large CsI ion, which will inhibit the transverse vibrations typically invoked to rationalise negative thermal expansion in PBAs,43 and which presumably also drive the phase transitions under compression. Consequently, the incorporation of CsI ions may be useful in applications where low strain and large resistance to phase transitions are desirable, e.g. in electrodes.44 Finally, CsMnCo(CN)6 shows an X-ray-induced lattice contraction, where further studies are required for mechanistic insight.
We are grateful to Diamond Light Source and ISIS Neutron and Muon Source for the provision of beamtimes CY30164 and 2010048 (data DOI: https://doi.org/10.5286/ISIS.E.RB2010048), respectively. We thank S. J. Cassidy (Oxford) and the I11 beamline staff for assistance with the data collection. HLBB acknowledges financial support from the Alexander von Humboldt Foundation. B. V. Lotsch (MPI-FKF) is gratefully acknowledged for the use of lab space, M.-L. Schreiber (MPI-FKF) and D. Ojwang (Uppsala) for ICP measurements, and C. L. Bull (ISIS) for useful discussions. Open Access funding provided by the Max Planck Society.
Author contributions
H. L. B. B., conceptualisation, investigation, formal analysis, writing; A. B. C., investigation, formal analysis, writing—review and editing; N. P. F., investigation, formal analysis, writing—review and editing; D. D., investigation; M. C., investigation; C. J. R., investigation, writing—review and editing.Conflicts of interest
There are no conflicts to declare.References
- K. Wang, P. Yan and M. Sui, Nano Energy, 2018, 54, 148–155 CrossRef CAS.
- J. Yang and Y. Xia, ACS Appl. Mater. Interfaces, 2016, 8, 1297–1308 CrossRef CAS PubMed.
- E. V. Boldyreva, V. Dmitriev and B. C. Hancock, Int. J. Pharm., 2006, 327, 51–57 CrossRef CAS PubMed.
- T. D. Bennett and A. K. Cheetham, Acc. Chem. Res., 2014, 47, 1555–1562 CrossRef CAS PubMed.
- K. W. Chapman, G. J. Halder and P. J. Chupas, J. Am. Chem. Soc., 2009, 131, 17546–17547 CrossRef CAS PubMed.
- S. C. McKellar and S. A. Moggach, Acta Crystallogr., Sect. B: Struct. Sci., 2015, B71, 587–607 CrossRef PubMed.
- M. J. Cliffe, J. A. Hill, C. A. Murray, F.-X. Coudert and A. L. Goodwin, Phys. Chem. Chem. Phys., 2015, 17, 11586–11592 RSC.
- S. Dissegna, P. Vervoorts, C. L. Hobday, T. Düren, D. Daisenberger, A. J. Smith, R. A. Fischer and G. Kieslich, J. Am. Chem. Soc., 2018, 140, 11581–11584 CrossRef CAS PubMed.
- A. B. Cairns, A. L. Thompson, M. G. Tucker, J. Haines and A. L. Goodwin, J. Am. Chem. Soc., 2012, 134, 4454–4456 CrossRef CAS PubMed.
- H. J. Buser, D. Schwarzenbach, W. Petter and A. Ludi, Inorg. Chem., 1977, 16, 2704–2710 CrossRef CAS.
- A. Ludi, H.-U. Güdel and M. Rüegg, Inorg. Chem., 1970, 9, 2224–2227 CrossRef CAS.
- G. Du and H. Pang, Energy Storage Mater., 2021, 36, 387–408 CrossRef.
- M. Verdaguer, A. Bleuzen, V. Marvaud, J. Vaissermann, M. Seuleiman, C. Desplanches, A. Scuiller, C. Train, R. Garde, G. Gelly, C. Lomenech, I. Rosenman, P. Veillet, C. Cartier and F. Villain, Coord. Chem. Rev., 1999, 190–192, 1023–1047 CrossRef CAS.
- D. Aguilà, Y. Prado, E. S. Koumousi, C. Mathonière and R. Clérac, Chem. Soc. Rev., 2016, 45, 203–224 RSC.
- S. Ayrault, B. Jimenez, E. Garnier, M. Fedoroff, D. J. Jones and C. Loos-Neskovic, J. Solid State Chem., 1998, 141, 475–485 CrossRef CAS.
- E. Ohara, T. Soejima and S. Ito, Inorg. Chim. Acta, 2021, 514, 120029 CrossRef CAS.
- J. Hu, H. Tao, M. Chen, Z. Zhang, S. Cao, Y. Shen, K. Jiang and M. Zhou, ACS Appl. Mater. Interfaces, 2022, 14, 12234–12242 CrossRef CAS PubMed.
- D. Wardecki, D. O. Ojwang, J. Grins and G. Svensson, Cryst. Growth Des., 2017, 17, 1285–1292 CrossRef CAS.
- V. K. Sharma, S. Mitra, A. Kumar, S. M. Yusuf, F. Juranyi and R. Mukhopadhyay, J. Phys.: Condens. Matter, 2011, 23, 446002 CrossRef CAS PubMed.
- S. H. Lapidus, G. J. Halder, P. J. Chupas and K. W. Chapman, J. Am. Chem. Soc., 2013, 135, 7621–7628 CrossRef CAS PubMed.
- H. L. B. Boström, A. B. Cairns, L. Liu, P. Lazor and I. E. Collings, Dalton Trans., 2020, 49, 12940–12944 RSC.
- C. S. Coates, C. A. Murray, H. L. B. Boström, E. M. Reynolds and A. L. Goodwin, Mater. Horiz., 2021, 8, 1446–1453 RSC.
- M. Gerstel, C. M. Deane and E. F. Garman, J. Synchrotron Radiat., 2015, 22, 201–212 CrossRef CAS PubMed.
- J. Christensen, P. N. Horton, C. S. Bury, J. L. Dickerson, H. Taberman, E. F. Garman and S. J. Coles, IUCrJ, 2019, 6, 703–713 CrossRef CAS PubMed.
- N. K. Fernando, A. B. Cairns, C. A. Murray, A. L. Thompson, J. L. Dickerson, E. F. Garman, N. Ahmed, L. E. Ratcliff and A. Regoutz, J. Phys. Chem. A, 2021, 125, 7473–7488 CrossRef CAS PubMed.
- H. L. B. Boström, I. E. Collings, D. Daisenberger, C. J. Ridley, N. P. Funnell and A. B. Cairns, J. Am. Chem. Soc., 2021, 143, 3544–3554 CrossRef PubMed.
- C. J. Howard, B. J. Kennedy and P. M. Woodward, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 463–471 CrossRef PubMed.
- A. M. Glazer, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, 28, 3384–3392 CrossRef CAS.
- H. L. B. Boström and W. R. Brant, J. Mater. Chem. C, 2022, 10, 13690–13699 RSC.
- J. Young and J. M. Rondinelli, J. Phys. Chem. Lett., 2016, 7, 918–922 CrossRef CAS PubMed.
- S. Vasala and M. Karppinen, Prog. Solid State Chem., 2015, 43, 1–36 CrossRef CAS.
- T. Matsuda, H. Tokoro, K. Hashimoto and S.-I. Ohkoshi, Dalton Trans., 2006, 5046–5050 RSC.
- T. Matsuda, J. E. Kim, K. Ohoyama and Y. Moritomo, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 172302 CrossRef.
- J. S. O. Evans, J. Chem. Soc., Dalton Trans., 1999, 3317–3326 RSC.
- C. S. Coates and A. L. Goodwin, Mater. Horiz., 2019, 6, 211–218 RSC.
- S. Kjeldgaard, I. Dugulan, A. Mamakhel, M. Wagemaker, B. Brummerstedt Iversen and A. Bentien, R. Soc. Open Sci., 2021, 8, 201779 CrossRef CAS PubMed.
- S. Adak, L. L. Daemen, M. Hartl, D. Williams, J. Summerhill and H. Nakotte, J. Solid State Chem., 2011, 184, 2854–2861 CrossRef CAS.
- K. W. Chapman, K. A. Beyer, H. Zhao and P. J. Chupas, CrystEngComm, 2013, 15, 9377–9381 RSC.
- Q. Gao, N. Shi, A. Sanson, Y. Sun, R. Milazzo, L. Olivi, H. Zhu, S. H. Lapidus, L. Zheng, J. Chen and X. Xing, Inorg. Chem., 2018, 57, 14027–14030 CrossRef CAS PubMed.
- Q. Gao, X. Shi, A. Venier, A. Carnera, Q. Huang, H. Wu, J. Chen, A. Sanson and E. Liang, Inorg. Chem., 2020, 59, 14852–14855 CrossRef CAS PubMed.
- K. W. Chapman, P. J. Chupas and C. J. Kepert, J. Am. Chem. Soc., 2006, 128, 7009–7014 CrossRef CAS PubMed.
- S. Margadonna, K. Prassides and A. N. Fitch, J. Am. Chem. Soc., 2004, 126, 15390–15391 CrossRef CAS PubMed.
- N. Shi, Q. Gao, A. Sanson, Q. Li, L. Fan, Y. Ren, L. Olivi, J. Chen and X. Xing, Dalton Trans., 2019, 48, 3658–3663 RSC.
- D. Asakura, M. Okubo, Y. Mizuno, T. Kudo, H. Zhou, K. Ikedo, T. Mizokawa, A. Okazawa and N. Kojima, J. Phys. Chem. C, 2012, 116, 8364–8369 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods; diffraction patterns and lattice parameters under variable pressure, temperature, and radiation; crystallographic data. See DOI: https://doi.org/10.1039/d2cp03754h |
| This journal is © the Owner Societies 2022 |




