Interplay of unique N⋯π pnicogen and H⋯π/H⋯O hydrogen bonding interactions in the heterodimers of nitromethane with acetylene and benzene as π-electron donors: experimental characterization at low temperatures under isolated conditions with computational corroboration†
Abstract
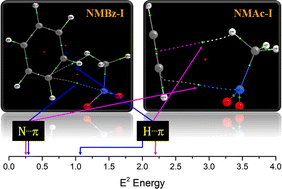
The existence of unique O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯π pnicogen bonding in combination with C–H⋯π/C–H⋯O hydrogen bonding interactions has been experimentally affirmed in the heterodimers of nitromethane with aliphatic (acetylene; NMAc) and aromatic (benzene; NMBz) π-electron donors at low temperatures under isolated conditions. The potential energy surfaces of NMAc and NMBz dimers have been probed for stationary points using ab initio and DFT computational methods. Three unique geometries for NMAc dimers were optimized, which were stabilized through mixed O
N⋯π pnicogen bonding in combination with C–H⋯π/C–H⋯O hydrogen bonding interactions has been experimentally affirmed in the heterodimers of nitromethane with aliphatic (acetylene; NMAc) and aromatic (benzene; NMBz) π-electron donors at low temperatures under isolated conditions. The potential energy surfaces of NMAc and NMBz dimers have been probed for stationary points using ab initio and DFT computational methods. Three unique geometries for NMAc dimers were optimized, which were stabilized through mixed O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯π pnicogen bonding and C–H⋯π/C–H⋯O hydrogen bonding interactions with varying strength. Within the Ar matrix at 12 K, only the C–H⋯π and C–H⋯O bound NMAc dimer was generated, while in N2, two geometries, one stabilized by pnicogen bonded O
N⋯π pnicogen bonding and C–H⋯π/C–H⋯O hydrogen bonding interactions with varying strength. Within the Ar matrix at 12 K, only the C–H⋯π and C–H⋯O bound NMAc dimer was generated, while in N2, two geometries, one stabilized by pnicogen bonded O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯π and C–H⋯π interactions and the other by C–H⋯π and C–H⋯O interactions, were produced. The NMBz dimer has only one structure stabilized by both O
N⋯π and C–H⋯π interactions and the other by C–H⋯π and C–H⋯O interactions, were produced. The NMBz dimer has only one structure stabilized by both O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯π pnicogen and C–H⋯π hydrogen bonding interactions that appears to be generated preferentially. The binding energies of both the dimers have the greatest contribution from electrostatics in both classes of NMAc and NMBz dimers, which is closely followed by dispersion forces in the case of NMBz. The increased proton affinity of benzene over acetylene appears to enhance the strength of C–H⋯π hydrogen bonds in NMBz while the O
N⋯π pnicogen and C–H⋯π hydrogen bonding interactions that appears to be generated preferentially. The binding energies of both the dimers have the greatest contribution from electrostatics in both classes of NMAc and NMBz dimers, which is closely followed by dispersion forces in the case of NMBz. The increased proton affinity of benzene over acetylene appears to enhance the strength of C–H⋯π hydrogen bonds in NMBz while the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯π pnicogen bonds remain quite similar in strength in both NMBz and NMAc dimers.
N⋯π pnicogen bonds remain quite similar in strength in both NMBz and NMAc dimers.


 Please wait while we load your content...
Please wait while we load your content...