Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Velocity map images of desorbing oxygen from sub-surface states of Rh(111)
Arved C.
Dorst
 ab,
Friedrich
Güthoff
ab,
Friedrich
Güthoff
 ab,
Daniel
Schauermann
ab,
Alec M.
Wodtke
ab,
Daniel
Schauermann
ab,
Alec M.
Wodtke
 ab,
Daniel R.
Killelea
ab,
Daniel R.
Killelea
 c and
Tim
Schäfer
c and
Tim
Schäfer
 *ab
*ab
aGeorg-August-Universität Göttingen, Institut für Physikalische Chemie, Tammannstr. 6, 37077 Göttingen, Germany. E-mail: tschaef4@gwdg.de
bMax-Planck-Institut für biophysikalische Chemie, Am Faßberg 11, 37077 Göttingen, Germany
cDepartment of Chemistry & Biochemistry, Loyola University Chicago, 1068 W. Sheridan Rd., Chicago, IL 60660, USA
First published on 9th September 2022
Abstract
We combine velocity map imaging (VMI) with temperature-programmed desorption (TPD) experiments to record the angular-resolved velocity distributions of recombinatively-desorbing oxygen from Rh(111). We assign the velocity distributions to desorption from specific surface and sub-surface states by matching the recorded distributions to the desorption temperature. These results provide insight into the recombinative desorption mechanisms and the availability of oxygen for surface-catalyzed reactions.
A Introduction
Surface-enhanced chemical reactivity is central for heterogeneously catalyzed processes that provide the foundation for the production of fine chemicals, materials, and pharmaceuticals.1 In such processes, when gas-phase reactants adsorb onto a surface, this interaction with the surface leads to bond weakening, thus facilitating the reaction. When elucidating the elementary reactions in heterogeneous catalysis, we normally consider only surface bound species involved in processes such as adsorption, dissociation, diffusion, reaction, and desorption; however, recent advances indicate that sub-surface species, which are absorbed at the selvedge of the catalyst, may also exert a strong influence on catalysis.2–5 These species not only provide an additional source of reactants, but they may also advantageously change the geometrical and electronic structure of the surface, or they even may provide altered reactivity due to the emergence of unique surface sites.6–8 Consequently, theoretical models that describe the chemical reactivity of such systems need to take the dynamics of sub-surface species into account and experiments providing data to develop suitable models are necessary.On many transition metal surfaces, oxygen molecules readily dissociate to form adsorbed oxygen atoms (Oad) and create characteristic adsorbate structures or induce surface reconstruction.9–11 At high O2 dosages or with aggressive oxidants like NO2 or gas-phase atomic oxygen (AO), sub-surface oxygen (Osub) may also form in the selvedge of the metal.12–15 In contrast to oxygen bound in metal oxides, Osub is dissolved in the metal and mobile under many real-world conditions. In recent years, O/Rh(111) has established itself as a benchmark system for the study of sub-surface oxygen formation; hence, much is already known. On dosing Rh(111) with O2 at room temperature, oxygen readily dissociates and saturates at an oxygen surface-coverage (θO) of 0.5 monolayers (ML, 1 ML = 1.6 × 1015 atoms cm−2)16 in a (2 × 1)-O adlayer.17 At these modest temperatures and low pressures, the rate of sub-surface oxygen formation is low. In contrast, when exposing Rh(111) to AO at elevated temperatures, sub-surface oxygen and single-layer RhO2 surface oxide domains are created in addition to the (2 × 1)-O adlayer.18 Interestingly, dosing Rh(111) at room temperature with AO produces no RhO2 surface oxide but results in the coexistence of the (2 × 2)-3O (θO = 0.75 ML) phase and the 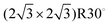 (θO = 0.67 ML) phase along with Osub.19–21 When heating up such a prepared surface to 700 K, the high oxygen density phases transform to the (2 × 1)-O adlayer with some oxide growth at the step edges, while the originally formed Osub remains below the surface. At an even higher temperature (∼800 K), sub-surface oxygen desorbs, which appears as a sharp desorption peak in the temperature-programmed desorption (TPD) experiment.18
(θO = 0.67 ML) phase along with Osub.19–21 When heating up such a prepared surface to 700 K, the high oxygen density phases transform to the (2 × 1)-O adlayer with some oxide growth at the step edges, while the originally formed Osub remains below the surface. At an even higher temperature (∼800 K), sub-surface oxygen desorbs, which appears as a sharp desorption peak in the temperature-programmed desorption (TPD) experiment.18
Still little is known about the microscopic details of the sub-surface oxygen desorption process. In general, defect sites and step edges likely provide a highly reactive environment on the (111) surface, potentially facilitating the emergence of Osub on the surface. However, a recent study on high index surfaces has shown that defects alone are not enough to explain the formation of Osub.22 Instead, an indication was observed that Osub emerges along the RhO2-metallic boundary, which forms when heating up O-adlayers at Rh(111). When sufficient amounts of RhO2 are formed, eruptive sub-surface oxygen desorption occurs.18 Once Osub is depleted, the oxide decomposes and the resulting surface-bound oxygen recombinatively desorbs.
Velocity map imaging (VMI) has recently been implemented to surface science studies in velocity resolved kinetics (VRK) experiments. Here, a pulsed molecular beam of reactants adsorbs at the surface on a microsecond timescale and the product flux is measured in front of the surface by ion imaging after laser ionization.24 This new technique has enabled a variety of studies resolving surface reaction kinetics with unprecedented resolution including CO oxidation on Pt, desorption kinetics of chiral molecules, H2 oxidation on Pt, and CO2 functionalization to formate on hydrogenated Pt.25–28
In this paper, we present velocity distributions of recombinatively-desorbing oxygen from Rh(111) obtained during TPD experiments in combination with VMI. Such distributions are valuable dynamical fingerprints of the microscopic processes involved in the desorption of sub-surface oxygen. Such fingerprints are used to test and refine theory.23 Specifically, we present results from a study combining VMI with TPD, allowing us to assign velocity distributions of desorption products to distinct peaks in the TPD spectrum with high temperature and velocity resolution. We observe hyperthermal velocity distributions for recombinatively-desorbing sub-surface oxygen as well as for recombinatively-desorbing surface oxygen from Rh(111) at T > 850 K. In contrast, O2 surface desorption around T = 750 K occurs sub-thermally indicating different desorption dynamics.
B Experimental
We performed the experiments under ultra-high vacuum (UHV) conditions at a background pressure of 3 × 10−10 Torr. The Rh(111) crystal (MaTecK, 99.99%, ∅ 10 mm, 2 mm thickness, and <0.1° orientation accuracy) is mounted on a copper sample holder and can be temperature controlled by cryogenic He cooling (ARS Cryo CS-204AB) and resistive heating between 30 K and 1300 K. The surface is cleaned by Ar–ion sputtering at 2000 V (Staib instruments IG-5-C), and subsequent annealing in an oxygen atmosphere (1.0 × 10−6 Torr) at 1300 K. Cleanliness of the surface and oxygen adsorption is checked by Meitner–Auger electron spectroscopy (Staib Instruments ESA-150) and X-ray photoelectron spectroscopy (Staib PSP CTX400). We dose the surface at room temperature with AO that we produce by using a microcapillary array beam doser29 and positioning the surface close to an Ir filament, through which we run (2.0–3.0) A at a chamber pressure of 1.0 × 10−6 Torr of O2. We do not see any indication of Ir in the Meitner–Auger spectrum after the dosing procedure.For VMI, we position the surface close to an ion-imaging detector as depicted in Fig. 1. While linearly increasing the temperature at the rate of 3 K s−1, we ionize the desorbing molecules with the focused (f = 300 mm) output of a regeneratively amplified femtosecond laser (800 nm, 35 fs, Solstice, Spectra-Physics). We record 10 images s−1 with 50 ionization events per image. The imaging detector consists of microchannel plates (MCPs, Topag MCP 56-15) and a phosphor screen (ProxiVision). Images are recorded with a CMOS camera (Basler ace acA1920-155 μm, (1920 × 1200) px). The MCPs are gated to the O2 time-of-flight by increasing the MCPs voltage by 450 V for a 200 ns time window. We bias the repeller to +3000 V and achieve VMI mode when attaching +1200 V to the ion lens in the time of flight tube. In this mode, ions with the same velocity components in v‖ and v⊥ are mapped on the same position on the phosphor screen. Synchronization of the experiment and data acquisition during heating of the crystal is achieved by a homebuilt LabVIEW program. For calibration of the VMI detector, we backfill the chamber with argon and non-resonantly ionize with a femtosecond laser pulse. The VMI image is fitted with a thermal Maxwell–Boltzmann distribution providing the pixel to velocity scaling factor of the detector as explained in detail in a previous publication.27 Additionally, we record conventional TPD spectra by linearly ramping up the sample temperature in front of a mass spectrometer (RGA-200, Stanford-Research-Systems).
C Results and discussion
Fig. 2 shows a conventional TPD spectrum of molecular oxygen desorption after dosing the surface with AO (with an Ir filament current of 2.0 A) for 300 s at Tsurf = 400 K. In agreement with previous studies, we observe two distinct features: sub-surface oxygen desorption causes a sharp peak around 815 K (α), while surface oxygen desorption is detected as an overall lift of the baseline signal over a wide temperature range at Tsurf > 850 K (β). Studies that combined scanning tunneling microscopy with TPD have shown that the β-peak corresponds to the decomposition of the (2 × 1)-O adlayer while the coverage decreases monotonically from 0.5 to 0. The α-peak corresponds to the desorption of subsurface oxygen accompanied by surface oxides and the (2 × 1)-O adlayer.18 | ||
| Fig. 2 Temperature-programmed desorption (TPD) spectrum of oxygen molecules after dosing Rh(111) with atomic oxygen at Tsurf = 400 K. We integrate the total ion signal for 32 amu while ramping up the surface temperature. We observe a distinct sharp desorption peak around 815 K (α) and a broad desorption feature at temperatures T > 850 K (β). The α-peak is caused by recombinatively-desorbing sub-surface oxygen atoms, and the β-peak by recombinatively-desorbing oxygen from the surface.18 The β-peak is broad and ranges up to 1300 K.21 Due to experimental limitations, we cannot detect ions up to this temperature. | ||
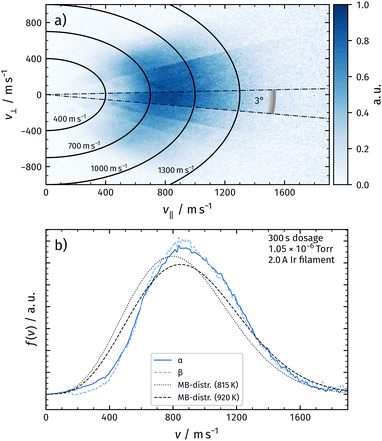
When recording the TPD spectrum with the velocity map imaging detector, we position the surface in front of the ion detector, and detect O2 desorption products by ionizing using a femtosecond laser (see Fig. 1). For mass resolution, we gate the ion time-of-flight to mass 32 amu while linearly ramping up the surface temperature at the rate of 3 K s−1. At this mass, the phosphor screen records VMI images for desorbing oxygen molecules. We record 30 images per Kelvin of the temperature ramp, which provide velocity distributions in the TPD spectrum with significantly less than 1 K resolution. Summing up all images obtained during the desorption temperatures of a distinct peak allows us to assign velocity distributions to the chosen peaks. The shaded regions in Fig. 2 indicate the temperatures at which VMI images had been summed up to obtain the velocity distributions of recombinatively-desorbing oxygen during α-peak and β-peak desorption.
We obtain angular distributions of desorbing molecules by moving the surface relative to the ionization region. VMI allows the measurement of angular distribution of desorption products as the desorption angle appears directly in the image. However, the geometrical setup of the experiment limits the sensitivity for large desorption angles due to the distance between the ionization region and the surface (see Fig. 3). With the used ion imaging setup, we obtain velocity map imaging conditions within a circular region of 10 mm in diameter. Considering the distance between the surface and laser ionization of 30 mm, it is clear that desorbing molecules at large angles with respect to the surface normal will not be detected in this region. These molecules can only be detected if the relative position of the detector and surface is changed. We therefore systematically move the surface position with respect to the detector as shown in Fig. 3 and record velocity map images as described above. For each position, we obtain an image providing desorption velocities at larger angles.
Fig. 4a shows the corresponding flux of desorbing O2 in the corresponding composite velocity map image for the α-peak. We extract the image from the raw data by subtracting the thermal background and converting density to flux by multiplying the signal with the corresponding velocity.27
We deduce velocity distributions for a certain desorption angle by integrating velocity segments of the image as depicted in Fig. 4a. In Fig. 4b, we show velocity distributions of the α-peak and the β-peak for an angle of 3° with respect to the surface normal. We do not observe significant differences between the two distributions. Velocity distributions obtained from desorbing molecules at different temperatures within one desorption peak look identical. We also plot a flux-weighted thermal Maxwell–Boltzmann distribution as in eqn (1) with Tsurf for comparison.
 | (1) |
From the integrated flux into a certain angular segment, we construct angular distributions from Fig. 4a, which can be fitted with a cos4(θ–4°) function. See Fig. 5, where we plot the results of the α-peak. We also calculate mean energies from the velocity distributions at various angles. The mean energy reduces at larger desorption angles. The dashed line indicates a cos2(θ–4°) function, which is expected for normal energy scaling.30 Identical results are obtained for the β-peak.
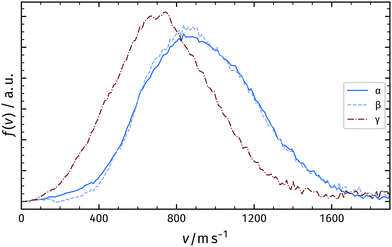
In a second experiment, we increased the atomic oxygen dosage by running 3.0 A instead of 2.0 A through the Ir filament. We again positioned the Rh crystal in front of the filament and dosed for 300 s. In Fig. 6a, we present the corresponding TPD spectrum. In addition to the desorption features observed with 2.0 A, we detect oxygen desorption at temperatures below the α-peak (γ).
Previous studies using atomic oxygen as the oxidizing agent did not detect the γ-peak.18,21 However, in contrast to those studies, the here employed experimental setup produces significantly higher AO fluxes towards the surface due to an elevated Ir filament temperature and the use of a microcapillary array beam doser. This probably leads to increased sub-surface oxygen generation. Fig. 6b shows the TPD spectrum after flash annealing the surface at 815 K, inducing the depletion of the γ-peak. The inset in Fig. 6 shows Meitner–Auger electron spectra before and after annealing at 815 K, indicating no significant change in the surface structure. We therefore exclude the formation of RhO2 at temperatures around the γ-peak desorption. If the γ-peak was from a phase transition, we would expect a sharp peak (like α); however, it is a broad feature and could be the result of the leakage of metastable sub-surface oxygen accompanied by the transition of a high-density oxygen surface phase to the (2 × 1)-O adlayer.18 We will continue to investigate the origin of the γ-peak in our subsequent studies.
We record velocity map images from the α-, β-, and γ-peaks as explained above. The velocity distributions of the α-peak and the β-peak are identical to the measurements with a low Ir current as shown in Fig. 3. The γ-peak desorption occurs with a distribution significantly shifted towards lower velocities compared to the distributions of the α- and β-peaks.
Table 1 summarizes the characteristics of the velocity distribution of different recombinatively-desorbing oxygen features in the TPD spectrum. The velocity distributions corresponding to desorption after sub-surface emergence (α-peak) and desorption after surface recombination at elevated temperatures (β-peak) resemble each other and are both hyperthermal as shown in Fig. 4. This might indicate a common intermediate surface state prior to desorption involving for instance RhO2 at the surface as suggested by Turano et al.18 In contrast, desorption of the γ-peak is sub-thermal and significantly shifted towards lower velocities indicating a different desorption mechanism.
| Peak | T/K | Type | v/m s−1 | v Th/m s−1 | E/eV |
|---|---|---|---|---|---|
| α | 815 | Osub | 940 | 865 | 0.131 |
| β | 910 | Oad | 955 | 914 | 0.136 |
| γ | 750 | ? | 766 | 830 | 0.0813 |
The here presented results are in qualitative agreement with the previous work of Gibson et al., who measured the hyperthermal time-of-flight (ToF) of desorbing sub-surface oxygen from Rh(111) and discussed their results in the framework of detailed balance.31 Considering the principles of detailed balance, hyperthermal velocity distributions indicate an activated dissociative adsorption process, presuming an equilibrium between dissociated atoms at the surface and desorbed gas–phase molecules. A well-studied reaction of this kind is the H2 dissociation on Cu(111), for which a large data set of ToF measurements exists after H atom permeation through the bulk.30,32–36 For this system, permeated hydrogen atoms reach local equilibrium at the surface, recombine, and desorb. The reactivity is well described by the principle of detailed balance, which provides state sensitive energy barriers for dissociation. On first glance, the O/Rh experiments presented here look similar, as oxygen atoms recombine and desorb from the surface. However, one should note that the employed universal detection method does not allow deducing quantum state resolved velocity distributions (e.g., v, J). Hence, the distributions are averaged over all quantum states that are populated in desorbing molecules from the surface.
In Fig. 8, we analyze energy distributions of α- and γ-peak desorption following the concepts of detailed balance. Panel (a) shows the energy distribution of recombinatively-desorbing sub-surface oxygen during the eruptive α-peak. Also shown is a flux-weighted thermal energy distribution at 815 K, which is clearly shifted towards lower energies. We fit the experimental data using eqn (2) which is the product of the flux-weighted thermal energy distribution and the sticking function:30
 | (2) |
 | (3) |
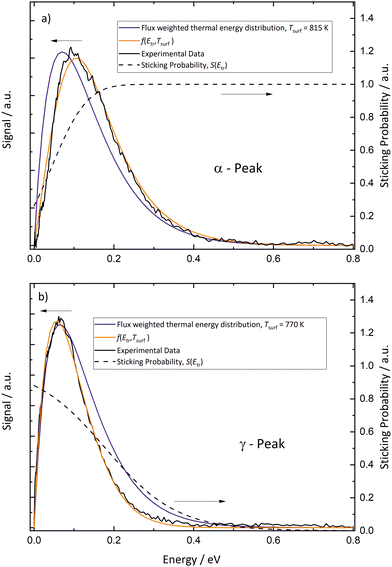 | ||
| Fig. 8 Panel (a) Energy distribution of recombinatively-desorbing sub-surface oxygen during the α-peak. Also displayed is a flux-weighted thermal energy distribution at 815 K. The measured distribution is clearly hyperthermal and can be modeled with the product of the flux-weighted thermal energy distribution and the sticking function (eqn (2), f(Etr, Tsurf)). Panel (b) The energy distribution of oxygen molecules desorbing during the γ-peak. The experimental data are clearly sub-thermal. The dashed lines show the sticking functions, which are fundamentally different for α-peak and γ-peak desorption. | ||
In contrast, desorption of the γ-peak occurs with an energy distribution which is clearly sub-thermal as shown in Fig. 8b. Consequently, the sticking probability decreases at elevated energies, shown as dashed line. We again employ eqn (2) to fit the data, but using another sticking function following the lines developed by Rettner et al.:44
 | (4) |
Conclusions
In this work, we have combined velocity map imaging (VMI) with temperature-programmed desorption (TPD) experiments to obtain velocity distributions of distinct peaks in the TPD spectrum of recombinatively-desorbing oxygen from Rh(111). The desorption of sub-surface oxygen after recombination at the surface (T = 815 K) exhibits a similar hyperthermal distribution to the desorption of surface oxygen at T > 900 K indicating a similar surface state prior to desorption. The velocity distribution of oxygen desorbing at T = 750 K is significantly shifted towards lower velocities and exhibits sub-thermal characteristics. We analyze the results using the principles of detailed balance to obtain energy dependent sticking functions. We identify different desorption dynamics on specific desorption peaks in the TPD spectrum. The α- and β-peak desorption occurs via activated recombination, while γ-peak desorption exhibits indication of an intermediate physisorbed molecular oxygen state prior to desorption. This study provides valuable data, on which models for sub-surface oxygen dynamics can be developed, tested, and refined, and also provides insight into the energetics of oxygen on metal surfaces relevant to heterogeneous catalysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. R. K wishes to acknowledge support from the National Science Foundation through awards CHE-1800291 and CHE-2155068 and ICASEC at University of Göttingen. T. S. acknowledges support from the Deutsche Forschungsgemeinschaft under Grant SCHA 1946/5-1 and INST 186/1302-1. Open Access funding provided by the Max Planck Society.Notes and references
- J. Ross, Heterogeneous Catalysis, Elsevier, Amsterdam, 2012 Search PubMed.
- M. Rebholz, R. Prins and N. Kruse, Surf. Sci., 1992, 269-270, 293–299 CrossRef CAS.
- A. von Oertzen, A. Mikhailov, H. H. Rotermund and G. Ertl, Surf. Sci., 1996, 350, 259–270 CrossRef CAS.
- N. M. H. Janssen, A. Schaak, B. E. Nieuwenhuys and R. Imbihl, Surf. Sci., 1996, 364, L555–L562 CrossRef CAS.
- J. Greeley and M. Mavrikakis, J. Phys. Chem. C, 2007, 111, 7992–7999 CrossRef CAS.
- Y. Xu, J. Greeley and M. Mavrikakis, J. Am. Chem. Soc., 2005, 127, 12823–12827 CrossRef CAS.
- S. T. Ceyer, Acc. Chem. Res., 2001, 34, 737–744 CrossRef CAS PubMed.
- K. D. Gibson, D. R. Killelea and S. J. Sibener, J. Phys. Chem. C, 2014, 118, 14977–14982 CrossRef CAS.
- C. Badan, R. G. Farber, Y. Heyrich, M. T. M. Koper, D. R. Killelea and L. B. F. Juurlink, J. Phys. Chem. C, 2016, 120, 22927–22935 CrossRef CAS.
- J. Derouin, R. G. Farber and D. R. Killelea, J. Phys. Chem. C, 2015, 119, 14748–14755 CrossRef CAS.
- D. H. Parker, M. E. Bartram and B. E. Koel, Surf. Sci., 1989, 217, 489–510 CrossRef CAS.
- J. Wider, T. Greber, E. Wetli, T. J. Kreutz, P. Schwaller and J. Osterwalder, Surf. Sci., 1998, 417, 301–310 CrossRef CAS.
- S. Schwegmann, H. Over, V. De Renzi and G. Ertl, Surf. Sci., 1997, 375, 91–106 CrossRef CAS.
- K. A. Peterlinz and S. J. Sibener, J. Phys. Chem., 1995, 99, 2817–2825 CrossRef CAS.
- K. D. Gibson, M. Viste, E. Sanchez and S. J. Sibener, J. Chem. Phys., 2000, 112, 2470–2478 CrossRef CAS.
- P. A. Thiel, J. T. Yates and W. H. Weinberg, Surf. Sci., 1979, 82, 22–44 CrossRef CAS.
- M. E. Turano, R. G. Farber, G. Hildebrandt and D. R. Killelea, Surf. Sci., 2020, 695, 121573 CrossRef CAS.
- M. E. Turano, E. A. Jamka, M. Z. Gillum, K. D. Gibson, R. G. Farber, W. Walkosz, S. J. Sibener, R. A. Rosenberg and D. R. Killelea, J. Phys. Chem. Lett., 2021, 12, 5844–5849 CrossRef CAS PubMed.
- J. Gustafson, A. Mikkelsen, M. Borg, E. Lundgren, L. Köhler, G. Kresse, M. Schmid, P. Varga, J. Yuhara, X. Torrelles, C. Quirós and J. N. Andersen, Phys. Rev. Lett., 2004, 92, 126102 CrossRef CAS PubMed.
- L. Köhler, G. Kresse, M. Schmid, E. Lundgren, J. Gustafson, A. Mikkelsen, M. Borg, J. Yuhara, J. N. Andersen, M. Marsman and P. Varga, Phys. Rev. Lett., 2004, 93, 266103 CrossRef PubMed.
- R. G. Farber, M. E. Turano, E. C. N. Oskorep, N. T. Wands, E. V. Iski and D. R. Killelea, J. Phys. Chem. C, 2017, 121, 10470–10475 CrossRef CAS.
- R. G. Farber, M. E. Turano, E. C. N. Oskorep, N. T. Wands, L. B. F. Juurlink and D. R. Killelea, J. Phys.: Condens. Matter, 2017, 29, 164002 CrossRef.
- D. J. Auerbach, J. C. Tully and A. M. Wodtke, Nat. Sci., 2021, 1, e10005 Search PubMed.
- D. J. Harding, J. Neugebohren, H. Hahn, D. J. Auerbach, T. N. Kitsopoulos and A. M. Wodtke, J. Chem. Phys., 2017, 147, 013939 CrossRef PubMed.
- J. Neugebohren, D. Borodin, H. W. Hahn, J. Altschäffel, A. Kandratsenka, D. J. Auerbach, C. T. Campbell, D. Schwarzer, D. J. Harding, A. M. Wodtke and T. N. Kitsopoulos, Nature, 2018, 558, 280–283 CrossRef CAS.
- D. Borodin, M. Schwarzer, H. W. Hahn, J. Fingerhut, Y. Wang, D. J. Auerbach, H. Guo, J. Schroeder, T. N. Kitsopoulos and A. M. Wodtke, Mol. Phys., 2021, 119, e1966533 CrossRef.
- J. Fingerhut, D. Borodin, M. Schwarzer, G. Skoulatakis, D. J. Auerbach, A. M. Wodtke and T. N. Kitsopoulos, J. Phys. Chem. A, 2021, 125, 7396–7405 CrossRef CAS PubMed.
- G. Westphal, J. Wega, R. E. A. Dissanayake and T. Schäfer, J. Chem. Phys., 2020, 153, 054707 CrossRef CAS PubMed.
- J. T. Yates Jr., Experimental Innovations in Surface Science, Springer, Cham, 2015 Search PubMed.
- H. A. Michelsen and D. J. Auerbach, J. Chem. Phys., 1991, 94, 7502–7520 CrossRef CAS.
- K. D. Gibson, J. I. Colonell and S. J. Sibener, Surf. Sci., 1995, 343, L1151–L1155 CrossRef CAS.
- G. Comsa and R. David, Surf. Sci., 1982, 117, 77–84 CrossRef CAS.
- H. A. Michelsen, C. T. Rettner and D. J. Auerbach, Surf. Sci., 1992, 272, 65–72 CrossRef CAS.
- C. T. Rettner, H. A. Michelsen, D. J. Auerbach and C. B. Mullins, J. Chem. Phys., 1991, 94, 7499–7501 CrossRef CAS.
- S. Kaufmann, Q. Shuai, D. J. Auerbach, D. Schwarzer and A. M. Wodtke, J. Chem. Phys., 2018, 148, 194703 CrossRef PubMed.
- Q. Shuai, S. Kaufmann, D. J. Auerbach, D. Schwarzer and A. M. Wodtke, J. Phys. Chem. Lett., 2017, 8, 1657–1663 CrossRef CAS PubMed.
- H. A. Michelsen, C. T. Rettner and D. J. Auerbach, Phys. Rev. Lett., 1992, 69, 2678–2681 CrossRef CAS PubMed.
- H. A. Michelsen, C. T. Rettner, D. J. Auerbach and R. N. Zare, J. Chem. Phys., 1993, 98, 8294–8307 CrossRef CAS.
- C. T. Rettner, H. A. Michelsen and D. J. Auerbach, J. Chem. Phys., 1995, 102, 4625–4641 CrossRef CAS.
- D. R. Killelea and A. L. Utz, Phys. Chem. Chem. Phys., 2013, 15, 20545–20554 RSC.
- A. C. Luntz, J. Chem. Phys., 2000, 113, 6901–6905 CrossRef CAS.
- M. Yan, Z.-Q. Huang, Y. Zhang and C.-R. Chang, Phys. Chem. Chem. Phys., 2017, 19, 2364–2371 RSC.
- E. J. Walter, S. P. Lewis and A. M. Rappe, J. Chem. Phys., 2000, 113, 4388–4391 CrossRef CAS.
- C. T. Rettner, E. K. Schweizer and C. B. Mullins, J. Chem. Phys., 1989, 90, 3800–3813 CrossRef CAS.
- T. Matsushima, Surf. Sci., 1985, 157, 297–318 CrossRef CAS.
| This journal is © the Owner Societies 2022 |