Development of antithrombotic peptides based on the molecular interactions between von Willebrand factor and GPIbα†
Abstract
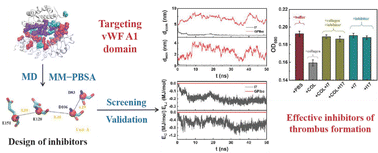
Binding of platelets on vascular endothelia at the damaged site using von Willebrand factor (vWF) as a bridge is of great significance for platelet adhesion and subsequent arterial thrombosis. Molecular interactions between vWF and a receptor on a platelet surface, GPIbα, were studied by molecular dynamics (MD) simulations and molecular mechanics–Poisson–Boltzmann surface area (MM–PBSA) analysis. Key amino acid residues were identified based on the contribution to the binding of GPIbα and the vWF A1 domain. A vWF-targeting inhibitor library with the amino acid sequence EXEXXDXD (where X represents any of the 20 natural amino acid residues) was then established based on the molecular interactions between GPIbα and the vWF A1 domain, subject to subsequent screening using docking, MD simulations, etc. Two efficient inhibitors including EGEPWDGD and EAEPWDPD were obtained, with experimental validation on their abilities to bind on the vWF and inhibiting platelet adhesion.


 Please wait while we load your content...
Please wait while we load your content...