Photochemistry of phosphenic chloride (ClPO2): isomerization with chlorine metaphosphite (ClOPO) and reduction by carbon monoxide†
Abstract
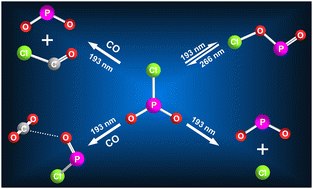
Phosphenic chloride (ClPO2) is an elusive congener of nitryl chloride (ClNO2). By high-vacuum flash pyrolysis of 2-chloro-1,3,2-dioxaphospholane in the gas phase, ClPO2 has been efficiently generated and subsequently isolated in cryogenic N2, Ar, and CO matrices (10 K) for a first time study on its photochemistry. Upon 193 nm laser irradiation, ClPO2 isomerizes to the novel chlorine metaphosphite (ClOPO) by initial cleavage of the Cl–P bond (→ ˙Cl + ˙PO2) with subsequent Cl–O bond formation inside the N2 and Ar matrix cages. The reverse transformation becomes feasible under further irradiation at 266 nm. This photochemistry is consistent with the observed absorptions of ClPO2 and ClOPO at 207 and 250 nm, respectively. When the photolysis was performed in solid CO ice, no isomerization occurs due to CO-trapping of the initially generated ˙Cl atoms by forming caged radical pair ClCO˙⋯˙PO2. Concomitantly, photolytic reduction of ClPO2 to ClPO by CO has been observed, yielding a weakly bonded molecular complex consisting of ClPO and CO2 bonded through short intermolecular C⋯O contact (2.910 Å). The characterization of ClPO, ClPO2, ClOPO, and the molecular complexes of ClPO2–CO and ClPO–CO2 using matrix-isolation IR and UV-vis spectroscopy is supported by the theoretical calculations at the B3LYP/6-311 + G(3df) level, and the photochemistry of ClPO2 is also compared with the revisited photochemistry of ClNO2 in the N2-matrix.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...