Modulating the intersystem crossing mechanism of anthracene carboxyimide-based photosensitizers via structural adjustments and application as a potent photodynamic therapeutic reagent†
Abstract
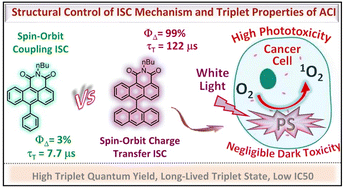
Herein, a series of compact anthracene carboxyimide (ACI) based donor–acceptor dyads were prepared by substituting bulky aryl moieties with various electron-donating ability to study the triplet-excited state properties. The ISC mechanism and triplet yield of the dyads were successfully tuned via structural manipulation. Efficient ISC (ΦΔ ≈ 99%) and long-lived triplet state (τT ≈ 122 μs) was observed for the orthogonal anthracene-labeled ACI derivative compared to the Ph-ACI and NP-ACI dyads, which showed fast triplet state decay (τT ≈ 7.7 μs). Femtosecond transient absorption study demonstrated the ultrafast charge separation (CS) and efficient charge recombination (CR) in the orthogonal dyads and ISC occurring via spin–orbit charge transfer (SOCT) mechanism (AN-ACI: τCS = 355 fs, τCR = 2.41 ns; PY-ACI: τCS = 321 fs, τCR = 1.61 ns), while in Ph-ACI and NP-ACI dyads triplet populate following the normal ISC channel (nπ* → ππ* transition), no CS was observed. We found that the attachment of suitable aryl donor moiety (AN- or PY-) to the ACI core can ensure the insertion of the intermediate triplet state, resulting in a small energy gap among charge separated state (CSS) and triplet state, which leads to efficient ISC in these derivatives. The SOCT-ISC-based AN-ACI dyad was confirmed to be a potent photodynamic therapeutic reagent; an ultra-low IC50 value (0.27 nM) that was nearly 214 times lower than that of the commercial Rose Bengal photosensitizer (57.8 nM) was observed.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...