Mutual effects between single-stranded DNA conformation and Na+–Mg2+ ion competition in mixed salt solutions†
Abstract
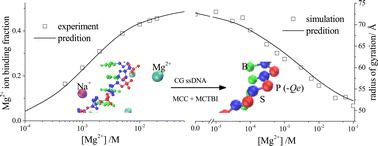
The ion-dependence of single-stranded DNA (ssDNA) conformational changes has attracted growing attention because of its biological and technological importance. Although single-species ion effects have been extensively explored, it is challenging to study the ssDNA conformational properties under mixed monovalent/divalent ion conditions due to the complications of ssDNA flexibility and ion–ion competition. In this study, we apply Langevin dynamics simulations to investigate mixed Na+/Mg2+ ion-dependent ssDNA conformations. The ssDNA structure is described using a coarse-grained model, in which the phosphate, base, and sugar of each nucleotide are represented by three different beads. A novel improvement in our simulation model is that mixed-salt-related electrostatic interactions are computed via combining Manning counterion condensation (MCC) theory with the Monte Carlo tightly bound ion (MCTBI) model. Based on this MCC-MCTBI combination, we report new empirical functions to describe the ion-concentration-dependent and ssDNA conformation/structure-dependent electrostatic effects. The calculation results relating to the ion binding properties and the simulation results relating to the ssDNA conformational properties are validated against experimental results. In addition, our simulation results suggest a quantitative relationship between the ssDNA conformation and Na+–Mg2+ competition; this in turn reveals their mutual impact in the ion atmosphere.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...