Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermodynamic study of crown ether–lithium/magnesium complexes based on benz-1,4-dioxane and its homologues†
Iklima
Oral
 a,
Fanny
Ott
a,
Fanny
Ott
 a and
Volker
Abetz
a and
Volker
Abetz
 *ab
*ab
aInstitute of Physical Chemistry, Universität Hamburg, Grindelallee 117, 20146 Hamburg, Germany
bHelmholtz-Zentrum Hereon, Institute of Membrane Research, Max-Planck-Straße 1, 21502 Geesthacht, Germany. E-mail: volker.abetz@hereon.de
First published on 28th April 2022
Abstract
The synthesis and characterization of benz-1,4-dioxane crown ethers (CEs) and some of its homologues are described and analyzed. The effect of added C-atom within the CE ring (increasing the hydrophobicity of the CE ring by increasing the number of CH2-units) on the Li+ and Mg2+ complexation within a liquid–liquid extraction (LLE) is investigated and thermodynamically analyzed. The complex stability constant K, the change of entropy ΔS and enthalpy ΔH, and the Gibbs energy ΔG are determined. The enhanced hydrophobicity of the CE ring results in stronger complexation stability of the Mg2+ complex, while the Li+ complexes are less favored. This effect mainly occurs due to the increased entropy term with improved hydrophobicity of the CE. These results indicate a stronger extraction of Li+ in Mg2+-containing aqueous resources if more hydrophilic CEs are used.
1. Introduction
Crown ethers (CE) are well-known for binding metal cations due to their cyclic cavities and electron donor features.1 CEs are macrocyclic ligands containing ethyleneoxy units, so-called polyethers. Since the name of these compounds is quickly complicated if larger rings are considered, Pedersen suggested the family name crown ether. This definition results from the molecular models and the ability to crown metal cations.2,3 The general structure of the CE is shown in Fig. 1, where X labels the total number of atoms and Y the total number of heteroatoms within the ring. If the CE arises from an arene catechol compound, the structure is named benzo-X-crown-Y.The interaction of a CE with a cation is mainly based on the interaction of charge (the metal cation) and dipole (donor atom within the CE ring), which can be influenced by the orientation of the donor atom, cavity size of the CE, type of donor atom (according to the HSAB principle), the substitution of electron-donating and withdrawing compounds and solvent.4 The large variability of the CE structures and their properties is why they are used in many different applications such as isotope separations,5 ion determination sensors,6 phase transfer catalysts,7 and ion transport through membranes.8,9
The application of CEs in solvent extraction is particularly noteworthy since it presents several benefits such as high selectivity, fast extraction rates, and easy application due to simple equipment on a laboratory and industrial scale. Especially in applications with polymers, improved adsorption properties and product recovery are more straightforward.10–12 The solvent extraction with CEs has been widely used to recover alkali and alkaline-earth metal ions such as Li+, Na+, K+, Mg2+, and Ca2+.10,13–16 Different cavity sizes, heteroatoms within the ring such as nitrogen or sulfur, substituents, and complex stoichiometries have been investigated.17–20 To remove the metal ions from the CE–metal complex, a temperature increase in the system releases the ions from this complex, as shown in the work of Warshawsky and Kahana.21
In this work, we synthesized different CEs based on benz-1,4-dioxane by increasing the cavity with an increased alkane chain (increasing the hydrophobicity of the CE). The effect of changing the ring size and increasing hydrophobicity surroundings on metal ion complexation is studied without incorporating other factors such as donor atoms or attached substituents. In this work, we will focus on the complexation stability of benz-1,4-dioxane-based CEs with the alkali metal ion Li+ and the alkaline-earth metal ion Mg2+. In terms of Li+ recovery from brines or seawater, the often-higher Mg2+ concentration leads to significant issues due to its similar behavior. Lithium and its compounds are widely used in industrial applications due to their physical and chemical properties and are therefore highly required.22 Especially in the electric vehicle industry, the demand has increased tremendously. The annual global lithium use was estimated to be 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 tons in 2019 and 2020, an 18% increase from 49
700 tons in 2019 and 2020, an 18% increase from 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 tons in 2018.23 Numerous CE adsorbers have been tested for Li+ recovery. For example, the complex stability with benzo-15-crown-5, 12-crown-4, and 14-crown-4 CEs adsorbers have shown excellent complexation properties with Li+; however, complexations with Mg2+ and Na+ were also possible.18,24–32 To selectively extract Li+ from aqueous resources, the complexation behavior of Li+ and the other metal ions is of great importance. Especially the complexation properties of Li+ and Mg2+ are of great interest since they have almost the same ion diameter in aqueous solution.33,34
100 tons in 2018.23 Numerous CE adsorbers have been tested for Li+ recovery. For example, the complex stability with benzo-15-crown-5, 12-crown-4, and 14-crown-4 CEs adsorbers have shown excellent complexation properties with Li+; however, complexations with Mg2+ and Na+ were also possible.18,24–32 To selectively extract Li+ from aqueous resources, the complexation behavior of Li+ and the other metal ions is of great importance. Especially the complexation properties of Li+ and Mg2+ are of great interest since they have almost the same ion diameter in aqueous solution.33,34
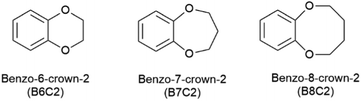
In this work the introduced benzo-6-crown-2 (B6C2), benzo-7-crown-2 (B7C2), and benzo-8-crown-2 (B8C2) structures (Fig. 2) are investigated regarding metal ion complex stability since, as far as we know, such adsorbers have not yet been studied. Moreover, the influence of the hydrophobic environment within the ring on the respective ions will be tested and discussed. The thermodynamic properties of Li+ and Mg2+ complexes will be further investigated by determining the enthalpy and entropy change during the complexation process.
2. Results and discussion
2.1. Synthesis of benz-1,4-dioxane crown ethers and homologues
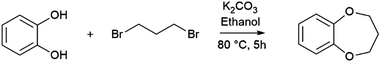
One of the most widely used methods for the syntheses of CEs is the Williamson ether synthesis, which was introduced by Alexander William Williamson.35 The Williamson ether synthesis is a variant of the nucleophilic substitution second-order reaction (SN2), characterized by a transition state. An alcohol, here: catechol 1, is converted into an dialcoholate 2 with the aid of a non-nucleophilic base K2CO3 (Fig. 3).36 It is worth mentioning that the alkoxides exhibit a higher nucleophilicity than alcohols, due to their increased electron density at the partially negatively charged oxygen atom, which accelerates the speed of the reaction. Due to their high base strength, ether synthesis is limited to the use of primary alkyl halides, since otherwise side-reactions would occur from the competing bimolecular elimination reaction (E2). It is worth mentioning that the use of phenoxides principally can lead to another side reaction, the C-alkylation. However, this can be prevented by using inert solvents such as benzene, toluene, xylene or polar aprotic solvents such as dimethylformamide (DMF) and dimethylsulfoxide (DMSO) to favor the oxygen alkylation.36 In the next step, the dialcoholate 2 reacts with a primary dihalogenide 3 (here: 1,3-dibromopropane) via a concerted, five-membered transition state 4 to form a new ether bond. An alkyl dibromide was chosen as an alkyl dihalide since the leaving capacity of the leaving group correlates with its ability to stabilize the negative charge and increases within the group F < Cl < Br < I due to increasing polarization capacity. In a further SN2 reaction, the oxygen nucleophile intramolecularly attacks the electrophilic center of the C–Br bond 5 and forms the seven-membered B7C2 CE ring 6 with two oxygen donor atoms. The synthesis of B8C2 follows the same reaction mechanism. For this, 1,4-dibromobutane was used as an alkyl dihalogenide. The synthetic routes were adapted/modified from the work of Y.-F. Ji et al.37 and H.-L. Zhu.38 Since these CEs have not been previously considered as ligands, sufficient analysis of these structures is performed by nuclear magnetic resonance (NMR) spectroscopy (Fig. 4) and infrared (IR) spectroscopy (Fig. S3 and S7, ESI†), differential scanning calorimetry (DSC) measurements (Fig. S4 and S8, ESI†), and mass spectrometry (MS) (Fig. 5). The 1H NMR data before purification of the products and the 13C NMR spectra are also documented in the ESI.† The data of the 1H- and 13C spectra are in good agreement with the data from the literature. To the best of our knowledge, the gas chromatography-MS (GC-MS), IR spectroscopy, and DSC data of these compounds have not been reported before.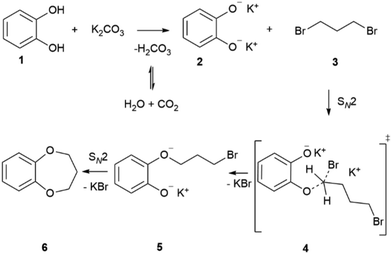 | ||
| Fig. 3 Illustration of the reaction mechanism of dipotassium catecholate with 1,3-dibromopropane under SN2 conditions to synthesize B7C2. | ||
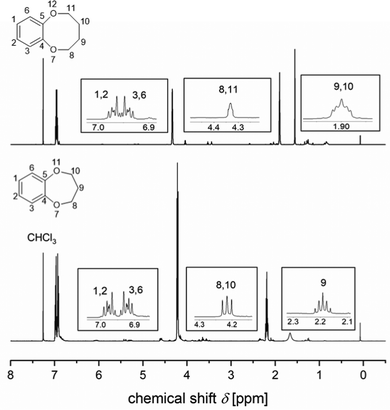 | ||
| Fig. 4 1H NMR spectrum of the B7C2 (bottom) and B8C2 (top). The spectrum was recorded in CDCl3 and shows the significant signals and their multiplicities on larger scale. | ||
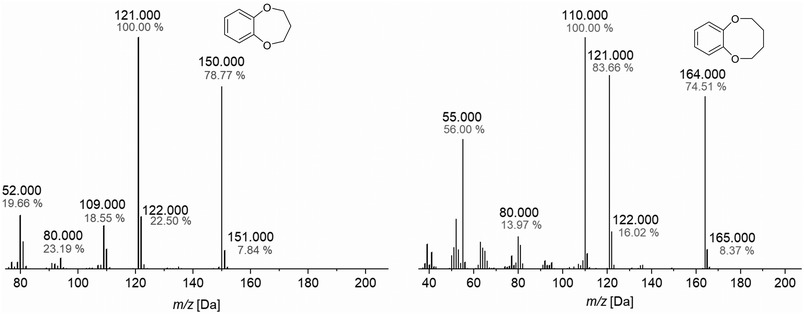
The molecular ion peak found at 151![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m/z agrees with the calculated molecular weight for approximately B7C2 (M = 151.178 g mol−1). The main fragmentation reactions consist of a benzyl cleavage. The delocalized π-electron system of the aromatic compound favors the formation of a stable benzyl ion (80
000 m/z agrees with the calculated molecular weight for approximately B7C2 (M = 151.178 g mol−1). The main fragmentation reactions consist of a benzyl cleavage. The delocalized π-electron system of the aromatic compound favors the formation of a stable benzyl ion (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m/z). The possible minor cleavage product is 1,3-cyclobutadiene (52
000 m/z). The possible minor cleavage product is 1,3-cyclobutadiene (52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m/z), formed by re-ionization. The peak at 121
000 m/z), formed by re-ionization. The peak at 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m/z shows a fragmentation based on a benzyl cleavage and hydrogen rearrangement, followed by McLafferty rearrangement and fragmentation. Instead of the McLafferty rearrangement, direct fragmentation of the radical cation is possible, whose quinoid cleavage product leads to the peak at 109
000 m/z shows a fragmentation based on a benzyl cleavage and hydrogen rearrangement, followed by McLafferty rearrangement and fragmentation. Instead of the McLafferty rearrangement, direct fragmentation of the radical cation is possible, whose quinoid cleavage product leads to the peak at 109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m/z. The GC-MS spectrum of the B8C2 shows a molecular ion peak at 164
000 m/z. The GC-MS spectrum of the B8C2 shows a molecular ion peak at 164![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m/z, which is in good agreement with the calculated molecular weight of M = 164
000 m/z, which is in good agreement with the calculated molecular weight of M = 164![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 m/z. The cleavage product of the McLafferty rearrangement at 121
080 m/z. The cleavage product of the McLafferty rearrangement at 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m/z has a lower intensity than B7C2. Thus, it can be assumed that the formation of this molecule ion peak decreases with the increasing ring size of the CE, which is most likely due to the steric relief caused by the chain breakage.
000 m/z has a lower intensity than B7C2. Thus, it can be assumed that the formation of this molecule ion peak decreases with the increasing ring size of the CE, which is most likely due to the steric relief caused by the chain breakage.
2.2. Pre-extraction experiments
In the following, the extraction properties of the newly synthesized B8C2 and B7C2 CEs and B6C2 (commercially available) with Li+ and Mg2+ in a liquid-liquid extraction (LLE) are investigated and the effect of ring size and hydrophobicity environment of the ring are discussed. To ensure that the CEs remain in the organic phase during the LLE, blank tests were performed. For this purpose, the CE was dissolved in an organic phase (here: DCM) and LiCl in the aqueous phase. The two-phase mixture was shaken vigorously, and the aqueous phase was subsequently analyzed by UV-vis spectroscopy. Since LiCl shows no absorption in the UV-vis spectrum, any visible signal should refer to the CE. Fig. S13 (ESI†) clearly shows that no absorption maximum is observed in the aqueous phase after extraction for the B7C2 and B8C2 CE structures. The B6C2 shows a small signal (black curve) below 1% and thus one can conclude that the CE–cation complex remains mainly in the organic phase.2.3. Determination of the thermodynamic complex constant K
The extractability of the metal salts by B6C2, B7C2, and B8C2 was investigated using a reference series of the respective cation. To determine the complexing properties of the CEs, the complex constant K was determined. To ensure UV-vis spectroscopic evaluation of salt complexation, counterions were used that allow π–π* excitation in the ultraviolet to visible spectral range through conjugated double bond systems and (anti-)auxochromic groups. The chosen metal salt dyes and their corresponding UV-vis spectrum can be found in the ESI† (Fig. S9–S12).For the LLE of the CE and cation dye, a molar ratio of CE to metal ion ranging from 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 11
1 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used. The reaction can be described as shown in eqn (1).
1 was used. The reaction can be described as shown in eqn (1).
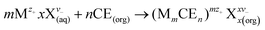 | (1) |
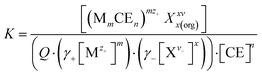 | (2) |
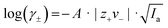 | (3) |
 | (4) |
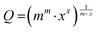 | (5) |
2.4. Extraction studies of B6C2, B7C2 and B8C2 with Li+ and Mg2+
In the following, the complex stability of B6C2, B7C2, and B8C2 at different concentrations with Li+ and Mg2+ at 25 °C is determined. The complexation constant K was calculated for a single and a sandwich-type cation–CE complexation. An illustration of the newly formed structure is exemplarily presented in Fig. 6 for the B7C2 with Li+ and Mg2+ and the corresponding anions. Since Li+ and Mg2+ are preferentially complexed in coordination number (CN) 4,41,42 a sandwich complex can be assumed (two CE ligands crown one metal ion). Furthermore, the cavity size of the CE might be too small for the metal ions to fit into the CE cavity.34 Therefore, the data presented here are calculated based on the assumption of a sandwich-type complexation.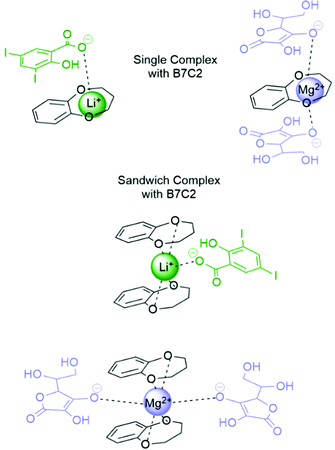 | ||
| Fig. 6 Schematic illustration of the B7C2–Li and Mg single and sandwich-type complex with corresponding anions. | ||
In the complexation process of metal ions from aqueous solutions, the formation of the hydration shell plays an essential role. The hydration shell must first be removed from metal ions to be complexed. The strength of the hydration shell depends on the charge and the size of the metal ion. The energy freed when one mole of ions undergoes hydration is called the hydration enthalpy ΔHhyd, which is the highest for Mg2+ (−1922 kJ mol−1), followed by Li+ (−521 kJ mol−1).43 The evaluated complex stability constants K for the molar ratio of CE to metal ion ranging from 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 11
1 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for single and sandwich-type complexation can be found in Fig. S14 and Table S1 (ESI†). A graphical illustration of the complexation constants K of the respective complexes is shown in Fig. 7 (values refer to the more likely sandwich-type complexation). It is already known that the substitution of the oxygen donor atoms with less electronegative atoms such as nitrogen favors the complexation of Mg2+ (as expected according to the HSAB principle). However, the influence of a more hydrophobic environment with a simple alkyl chain has not been studied yet. The introduction of a more hydrophobic ring shows a stronger complex stability constant K for Mg2+ which is indicated by the following trend B8C2 = B7C2 > B6C2. For Li+, on the other hand, the trend is vice versa showing an increased log
1 for single and sandwich-type complexation can be found in Fig. S14 and Table S1 (ESI†). A graphical illustration of the complexation constants K of the respective complexes is shown in Fig. 7 (values refer to the more likely sandwich-type complexation). It is already known that the substitution of the oxygen donor atoms with less electronegative atoms such as nitrogen favors the complexation of Mg2+ (as expected according to the HSAB principle). However, the influence of a more hydrophobic environment with a simple alkyl chain has not been studied yet. The introduction of a more hydrophobic ring shows a stronger complex stability constant K for Mg2+ which is indicated by the following trend B8C2 = B7C2 > B6C2. For Li+, on the other hand, the trend is vice versa showing an increased log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K in the following trend: B6C2 ≈ B7C2 > B8C2 and thus decreasing with higher hydrophobicity of the ring. According to the HSAB principle Li+ (hardness η = 35.12) is a harder base than Mg2+ (η = 32.55),44,45 which could explain the obtained trend.
K in the following trend: B6C2 ≈ B7C2 > B8C2 and thus decreasing with higher hydrophobicity of the ring. According to the HSAB principle Li+ (hardness η = 35.12) is a harder base than Mg2+ (η = 32.55),44,45 which could explain the obtained trend.
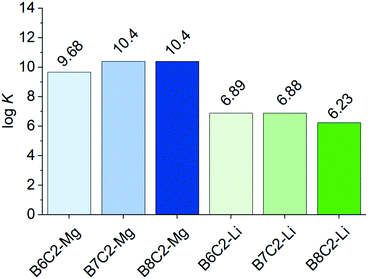 | ||
Fig. 7 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K of the CE K of the CE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal ion complexes with B6C2, B7C2, and B8C2 with Mg2+ and Li+ at a constant molar ratio of CE metal ion complexes with B6C2, B7C2, and B8C2 with Mg2+ and Li+ at a constant molar ratio of CE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal ion of 14 metal ion of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
2.5. Temperature-dependent extraction studies of B6C2, B7C2 and B8C2 with Li+ and Mg2+
In the following, enthalpic and entropic values are determined experimentally by temperature-dependent LLE to clarify the differences of complex stability for the B6C2, B7C2 and B8C2 CEs more precisely. The relation between the complex constant K and temperature is described by the Van’t Hoff equation (VH, eqn (6)) assuming that ΔH and ΔS are temperature independent.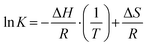 | (6) |
The VH equation arises from the combination of the Gibbs energy of reaction and the Gibbs energy isotherm equation, which is described in eqn (7) and (8). R labels the molar gas constant (8.314 J mol−1 K−1).
| ΔG = ΔH − TΔS | (7) |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K | (8) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K is a linear function of the reciprocal temperature 1/T. The plot of the values results in a linear regression, where the slope gives
K is a linear function of the reciprocal temperature 1/T. The plot of the values results in a linear regression, where the slope gives 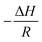 and the intercept
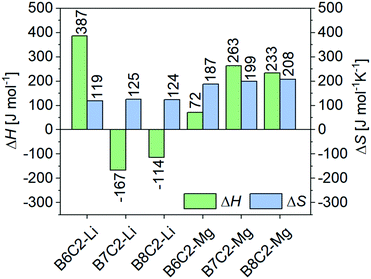
and the intercept 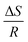 . The VH plots are shown in Fig. S15 (ESI†) and the corresponding values to determine ΔS and ΔH can be found in Table S2 (ESI†). The data of the enthalpy and entropy changes ΔH and ΔS after complexation of B6C2, B7C2, and B8C2 with Li+ and Mg2+ are shown in Fig. 8. The green bar labels ΔH and the blue bar labels ΔS.
. The VH plots are shown in Fig. S15 (ESI†) and the corresponding values to determine ΔS and ΔH can be found in Table S2 (ESI†). The data of the enthalpy and entropy changes ΔH and ΔS after complexation of B6C2, B7C2, and B8C2 with Li+ and Mg2+ are shown in Fig. 8. The green bar labels ΔH and the blue bar labels ΔS.
Generally, an enthalpy gain occurs if new interactions proceed between the metal ion and the donor atoms of the CE. On the other hand, the anion interacts less pronounced in the organic phase compared to the aqueous phase, resulting in an enthalpy loss.39 The enthalpy change ΔH is highest for the B6C2 for Li+ and decreases rapidly when the ring size as well as the hydrophobicity increases. In fact, negative values are obtained for the B7C2 and B8C2 CEs, which might be due to the weaker ΔHhyd of Li+ compared to Mg2+, making the desolvation less energy-consuming and, therefore, enthalpic change more favorable. However, this is not the case for B6C2–Li. The energy contributions are significantly higher for the Mg2+ complexes, which is most likely due to the stronger bonding of the water molecules to the Mg2+ in the aqueous phase. In addition, two anions have to be freed from the hydration shell compared to the Li complex to form the CE–Mg complex (compare with Fig. 6). In addition, ΔH increases for the more hydrophobic CE rings such as B7C2 and B8C2 in comparison to B6C2.
The change of Gibbs energy of a system is an interplay between ΔH and ΔS. The change of entropy ΔS is almost identical for Li+ complexes and increases only slightly with increasing hydrophobicity of the CE. On the other hand, ΔS increases with growing hydrophobicity for the CE–Mg complexes, which is most likely the reason for the higher complex stability K (Fig. 7). The higher ΔS of Mg2+ complexes compared to Li+ ones most-probably result from the released water molecules, which were strongly bound to the Mg2+ ion before complexation. As previously mentioned, the hydration enthalpy ΔHhyd is almost four times larger than the enthalpy that would be required to desolvate lithium. Accordingly, the water molecules are much more strongly bound to the Mg2+. In addition, in the case of the Mg2+ complexes, two anions are attached to the complex so that twice the amount of water molecules are released. In contrast, the formation of complexes in the organic phase also leads to entropy loss since the CEs lose mobility. The larger the complex, the greater this loss. The entropy loss is thus more significant for a sandwich-type complex compared to a single complex. The change in entropy ΔS is, therefore an interplay between the gain in entropy due to the release of water molecules in the aqueous phase and the loss of entropy due to the binding of the ligands and anions to the metal ion in the organic phase.
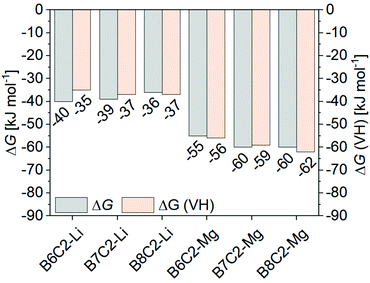
To describe the energy of a system and its voluntariness of the complex formation, the Gibbs energy ΔG is used. Fig. 9 shows the calculated ΔG values calculated according to eqn (7) by performing extraction at room temperature (grey bar) and by eqn (8) conducting temperature-dependent extractions using the VH equation (ΔG (VH), orange bar). Both ΔG values agree well with each other. All reactions are exergonic and proceed voluntarily, with the Mg2+ complexes being more favorable. This effect is more pronounced with increasing hydrophobicity of the CE ring.
The CEs prepared in this work are new adsorbers for lithium isolation that have not been previously considered in the group of benz-1,4-dioxane homologues with an increasing alkane chain. In addition, CE B7C2 in particular is less expensive in comparison to the commercially available 12-crown-4 and 15-crown-4 CEs that are commonly used for lithium isolation and since it is less soluble in water, the yield of complexed lithium is larger.
3. Conclusions
B7C2 and B8C2 CEs were synthesized and compared with B6C2 in LLE to investigate the Li+ and Mg2+ extractability from aqueous solution. Thermodynamic parameters such as the complex stability constant K, the Gibbs energy ΔG (calculated from room temperature and temperature-dependent extractions), and the change in entropy ΔS and enthalpy ΔH were determined and discussed. The increase of the number of C atoms within the CE ring (higher hydrophobicity) leads to higher Mg2+ complex stability, while the Li+ complex stability is decreased. The higher stability of Mg2+ complexes is most likely based on a greater change in change of entropy ΔS with increasing hydrophobicity. In summary, the data reported here show that the effect of hydrophobicity has brought us much closer to the selective extraction of Li+ over Mg2+ and that these results can further narrow the choice of future adsorbents for lithium extraction to find a suitable material.4. Experimental
4.1 Materials
1,4-Benzdioxane (98%, Sigma-Aldrich, Schnelldorf, Germany), (+)-Mg-(L)-ascorbate (98%, Sigma-Aldrich, Schnelldorf, Germany), lithium 3,5-diiodosalicylate (>99%, LIS, Sigma-Aldrich, Schnelldorf, Germany), potassium carbonate (99%, Sigma-Aldrich, Schnelldorf, Germany), catechol (99%, Sigma-Aldrich, Schnelldorf, Germany), dichloromethane (DCM) (>99%, Acros, Schwerte, Germany), petrol ether (technical grade), ethyl acetate (technical grade), 1,3-dibromopropane (>99%, Sigma-Aldrich, Schnelldorf, Germany), 1,4-dibromobutane (>99%, Sigma-Aldrich, Schnelldorf, Germany), 2-propanol (>99%, Sigma-Aldrich, Schnelldorf, Germany) n-hexane (95%, VWR Chemicals, Darmstadt, Germany), chloroform-d1 (>99%, EurisoTop, Saarbrücken, Germany) were used as received without further purification unless noted otherwise. Deionized water was purified with a Milli-Q® integral water purification system.4.2 Methods
4.3 Synthetic procedures
In this work, the preparation of the crown ethers was carried out in a modified form according to a prescription of Huang et al.37 The reactions took place under a nitrogen atmosphere.Catechol (5.6 g, 51 mmol, 1.0 eq.), 1,3-dibromopropane (14 g, 7.1 mL, 69 mmol, 1.4 eq.) and potassium carbonate (25 g, 0.18 mol, 3.5 eq.) were dissolved in ethanol (27% (v/v), 150 mL). The green mixture was heated under reflux for 5 h at 80 °C and turned into an orange mixture. The reaction was cooled to room temperature and filtrated under reduced pressure. The crude product was purified two times with column chromatography on silica gel with a mobile phase of petrol ether and ethyl acetate (30
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and (25
1 v/v) and (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The product was obtained as a colorless oil (4.1 g, 54%). 1H NMR (500 MHz, CDCl3, 25 °C): δ [ppm] = 7.00–6.91 (m, Ar-H, 4H), 4.22 (t, 3J (H, H) = 3 Hz, O–CH2, 4H), 2.19 (quint., 3J (H,H) = 3 Hz, –CH2, 2H); 13C NMR (500 MHz, CDCl3, 25 °C): δ [ppm] = 152 (C4), 124 (C4–CH–CH), 122 (C4–CH–CH), 70.9 (O–CH2–), 32.2 (–CH2); GC-MS (EI): m/z (%): [M]+ calcd for C9H10O2+: 151.08, found 151.00 (8) [M+], 121.00 (100) [C7H7O2+], 109.00 (19) [C6H4O2+], 80.00 (23) [C6H6+], 51.02 (20) [C4H3+]; IR (KBr):
1 v/v). The product was obtained as a colorless oil (4.1 g, 54%). 1H NMR (500 MHz, CDCl3, 25 °C): δ [ppm] = 7.00–6.91 (m, Ar-H, 4H), 4.22 (t, 3J (H, H) = 3 Hz, O–CH2, 4H), 2.19 (quint., 3J (H,H) = 3 Hz, –CH2, 2H); 13C NMR (500 MHz, CDCl3, 25 °C): δ [ppm] = 152 (C4), 124 (C4–CH–CH), 122 (C4–CH–CH), 70.9 (O–CH2–), 32.2 (–CH2); GC-MS (EI): m/z (%): [M]+ calcd for C9H10O2+: 151.08, found 151.00 (8) [M+], 121.00 (100) [C7H7O2+], 109.00 (19) [C6H4O2+], 80.00 (23) [C6H6+], 51.02 (20) [C4H3+]; IR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) [cm−1] = 3037 (w), 2950 (m), 2866 (w), 1580 (m), 1490 (s), 1246 (s), 1048 (s), 751 (s).
[cm−1] = 3037 (w), 2950 (m), 2866 (w), 1580 (m), 1490 (s), 1246 (s), 1048 (s), 751 (s).
Catechol (6.7 g, 61 mmol, 1.0 eq.) was dissolved in 2-propanol (14% (v/v), 170 mL). After adding potassium carbonate (29.5 g, 214 mmol, 3.5 eq.), 1,4-dibromobutane (7.65 mL, 14 g, 64 mmol, 1.0 eq.) was added dropwise to the reaction mixture. The turbid reaction mixture was heated under reflux for 15 h at 90 °C. The red solution was cooled to room temperature and subsequently extracted 5 times with dichloromethane. The eluate was washed with water and brine. Any residue of solvent was removed under reduced pressure. The orange residue was purified two times with column chromatography on silica gel with a mobile phase of n-hexane and ethyl acetate (8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and (10
1 v/v) and (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The product was obtained as a light-yellow-colored oil (0.3 g, 3%). 1H NMR (500 MHz, CDCl3, 25 °C): δ [ppm] = 6.98–6.93 (m, Ar-H, 4H), 4.34–4.32 (m, O–CH2, 4H), 1.91–1.89 (m, –CH2, 4H); 13C NMR (500 MHz, CDCl3, 25 °C): δ [ppm] = 150 (C4), 124 (C4–CH–CH), 123 (C4–CH–CH), 72.8 (O–CH2–), 27.1 (–CH2); GC-MS (EI): m/z (%): [M]+ calcd for C10H12O2+: 165.09, found 165.00 (8) [M+], 121.00 (84) [C7H7O2+], 110.00 (100) [C6H4O2+], 80.00 (14) [C6H6+], 55.00 (56) [C4H3+]; IR (KBr):
1 v/v). The product was obtained as a light-yellow-colored oil (0.3 g, 3%). 1H NMR (500 MHz, CDCl3, 25 °C): δ [ppm] = 6.98–6.93 (m, Ar-H, 4H), 4.34–4.32 (m, O–CH2, 4H), 1.91–1.89 (m, –CH2, 4H); 13C NMR (500 MHz, CDCl3, 25 °C): δ [ppm] = 150 (C4), 124 (C4–CH–CH), 123 (C4–CH–CH), 72.8 (O–CH2–), 27.1 (–CH2); GC-MS (EI): m/z (%): [M]+ calcd for C10H12O2+: 165.09, found 165.00 (8) [M+], 121.00 (84) [C7H7O2+], 110.00 (100) [C6H4O2+], 80.00 (14) [C6H6+], 55.00 (56) [C4H3+]; IR (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) [cm−1] = 3069 (w), 2931 (m), 2870 (w), 1578 (m), 1490 (s), 1242 (s), 972 (s), 753 (s).
[cm−1] = 3069 (w), 2931 (m), 2870 (w), 1578 (m), 1490 (s), 1242 (s), 972 (s), 753 (s).
4.4 Liquid–liquid extraction
The organic phase was prepared by dissolving appropriate amounts of CE (m ≈ 3 mg) in 3 mL dichloromethane (concentration of the CE cCE ranging from 6.0 × 10−3 to 8.0 × 10−3 mol L−1). The aqueous phase was prepared by diluting salts in water at CE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) salt molar ratio between 25
salt molar ratio between 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 11
1 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (concentration of the salts cSalt ranging from 2.0 × 10−4 to 8.0 × 10−4 mol L−1). For all extraction experiments, the volume ratio of dichloromethane–water was 1
1 (concentration of the salts cSalt ranging from 2.0 × 10−4 to 8.0 × 10−4 mol L−1). For all extraction experiments, the volume ratio of dichloromethane–water was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The extraction proceeded by vigorously shaking the vial for 45 min at 500 rpm at specific temperatures. Phase separation was guaranteed by waiting 30 min after extraction. The aqueous phase was adequately diluted, and the remaining concentration of the metal ion was measured by a UV-vis spectrometer.
1. The extraction proceeded by vigorously shaking the vial for 45 min at 500 rpm at specific temperatures. Phase separation was guaranteed by waiting 30 min after extraction. The aqueous phase was adequately diluted, and the remaining concentration of the metal ion was measured by a UV-vis spectrometer.
4.5 Temperature-dependent liquid–liquid extraction
Temperature-dependent extractions were performed with a constant molar concentration of CE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) salt = 14
salt = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The extraction conditions were carried out analogously to the conditions described above, with only the temperature being changed in a range between 10 and 30 °C. For the temperature setting, a cooling and heating thermomixer MKR13 (DITABIS) was used, which has a temperature accuracy of ±0.1 °C. The thermomixer was used for the temperature adjustment. To additionally determine the temperature in the solution, a water sample was placed into the thermomixer in which an additional thermometer permanently measured the temperature.
1. The extraction conditions were carried out analogously to the conditions described above, with only the temperature being changed in a range between 10 and 30 °C. For the temperature setting, a cooling and heating thermomixer MKR13 (DITABIS) was used, which has a temperature accuracy of ±0.1 °C. The thermomixer was used for the temperature adjustment. To additionally determine the temperature in the solution, a water sample was placed into the thermomixer in which an additional thermometer permanently measured the temperature.
Author contributions
I. O. and V. A.: conceptualization. I. O. and F. O.: methodology, investigation, data curation, formal analysis, and validation; V. A.: supervision and resources; I. O.: writing the original draft; I. O and V. A.: review and editing of the original draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the NMR spectroscopy and mass spectrometry division of the Department of Chemistry at the Universität Hamburg, and Prof. Dr Weller for providing access to the DSC instrument.Notes and references
- J. C. Shen, W. L. Jiang, W. Di Guo, Q. Y. Qi, D. L. Ma, X. Lou, M. Shen, B. Hu, H. B. Yang and X. Zhao, A rings-in-pores net: Crown ether-based covalent organic frameworks for phase-transfer catalysis, Chem. Commun., 2020, 56, 595–598 RSC.
- C. J. Pedersen, Cyclic Polyethers and Their Complexes with Metal Salts, J. Am. Chem. Soc., 1967, 89, 7017–7036 CrossRef CAS.
- G. W. Gokel, W. M. Leevy and M. E. Weber, Crown ethers: Sensors for ions and molecular scaffolds for materials and biological models, Chem. Rev., 2004, 104, 2723–2750 CrossRef CAS PubMed.
- A. Boda, S. Musharaf Ali, H. Rao and S. K. Ghosh, Ab initio and density functional theoretical design and screening of model crown ether based ligand (host) for extraction of lithium metal ion (guest): Effect of donor and electronic induction, J. Mol. Model., 2012, 18, 3507–3522 CrossRef CAS PubMed.
- F. Yan, H. Liu, H. Pei, J. Li, Z. Cui and B. He, Polyvinyl alcohol-graft-benzo-15-crown-5 ether for lithium isotopes separation by liquid–solid extraction, J. Radioanal. Nucl. Chem., 2017, 311, 2061–2068 CrossRef CAS.
- M. Kamenica, R. R. Kothur, A. Willows, B. A. Patel and P. J. Cragg, Lithium ion sensors, Sensors, 2017, 17, 2430 CrossRef PubMed.
- P. E. Stott, J. S. Bradshaw and W. W. Parish, Modified Crown Ether Catalysts. 3. Structural Parameters Affecting Phase Transfer Catalysis by Crown Ethers and a Comparison of the Effectiveness of Crown Ethers to That of Other Phase Transfer Catalysts, J. Am. Chem. Soc., 1980, 102, 4810–4815 CrossRef CAS.
- S. Tas, B. Zoetebier, M. A. Hempenius, G. J. Vancso and K. Nijmeijer, Monovalent cation selective crown ether containing poly(arylene ether ketone)/SPEEK blend membranes, RSC Adv., 2016, 6, 55635–55642 RSC.
- A. Casadellà, A. H. Galama, O. Schaetzle and K. Loos, Effect of Diffusion and Migration on the Selectivity of a Polymer Inclusion Membrane Containing Dicyclohexano-18-crown-6, Macromol. Chem. Phys., 2016, 217, 1600–1606 CrossRef.
- I. Oral and V. Abetz, A Highly Selective Polymer Material using Benzo-9-Crown-3 for the Extraction of Lithium in Presence of Other Interfering Alkali Metal Ions, Macromol. Rapid Commun., 2021, 42, 2000746 CrossRef CAS PubMed.
- I. Oral, L. Grossmann, E. Fedorenko, J. Struck and V. Abetz, Synthesis of Poly(methacrylic acid)-block-Polystyrene Diblock Copolymers at High Solid Contents via RAFT Emulsion Polymerization, Polymers, 2021, 13, 3675 CrossRef CAS PubMed.
- I. Oral and V. Abetz, Improved alkali metal ion capturing utilizing crown ether-based diblock copolymers in a sandwich-type complexation, Soft Matter, 2022, 18, 934–937 RSC.
- K. Kimura, S. Kanokogi and M. Yokoyama, Extraction Spectrophotometry of Lithium Ions Based on Cation-and Photo-Induced Isomerization of Crowned Spirobenzopyran, Anal. Sci., 1996, 12, 399–403 CrossRef CAS.
- Y. Tian, W. Chen, Z. Zhao, L. Xu and B. Tong, Interaction and selectivity of 14-crown-4 derivatives with Li +, Na +, and Mg2+ metal ions, J. Mol. Model., 2020, 26, 67 CrossRef CAS PubMed.
- K. Sambe, N. Hoshino, T. Takeda, T. Nakamura and T. Akutagawa, Dynamics and Structural Diversity of Li + (Crown Ether) Supramolecular Cations in Electrically Conducting Salts, J. Phys. Chem. C, 2020, 124, 13560–13571 CrossRef CAS.
- Y. Katayama, K. Nita, M. Ueda, H. Nakamura, M. Takagi and K. Ueno, Synthesis of chromogenic crown ethers and liquid-liquid extraction of alkali metal ions, Anal. Chim. Acta, 1985, 173, 193–209 CrossRef CAS.
- R. E. C. Torrejos, G. M. Nisola, S. H. Min, J. W. Han, S. Koo, K. J. Parohinog, S. Lee, H. Kim and W. J. Chung, Aqueous Synthesis of 14-15-Membered Crown Ethers with Mixed O, N and S Heteroatoms: Experimental and Theoretical Binding Studies with Platinum-Group Metals, ChemPlusChem, 2019, 84, 210–221 CrossRef CAS PubMed.
- Y. Inoue, T. Hakushi, Y. Liu and L. H. Tong, Molecular Design of Crown Ethers. 12. Complexation Thermodynamics of 12- to 16-Crown-4: Thermodynamic Origin of High Lithium Selectivity of 14-Crown-4, J. Org. Chem., 1993, 58, 5411–5413 CrossRef CAS.
- Y. Tobe, Y. Tsuchiya, H. Iketani, K. Naemura, K. Kobiro, M. Kaji, S. Tsuzuki and K. Suzuki, Synthesis and lithium ion selectivity of 14-crown-4 derivatives having bulky subunits: cis and trans isomers of 2-phenylcyclohexano-14-crown-4,2,3-diphenylcyclohexano-14-crown-4 and 2,3-di-(1-adamantyl)-14-crown-4, J. Chem. Soc., Perkin Trans. 1, 1998,(3), 485–494 RSC.
- I. Oral, S. Tamm, C. Herrmann and V. Abetz, Lithium Selectivity of Crown Ethers: The Effect of Heteroatoms and Cavity Size, Sep. Purif. Technol., 2022, 121142 CrossRef.
- A. Warshawsky and N. Kahana, Temperatre-Regulated Release of Alkali Metal Salts from Novel Polymeric Crown Ether Complexes, J. Am. Chem. Soc., 1982, 104, 2663–2664 CrossRef CAS.
- G. Martin, L. Rentsch, M. Höck and M. Bertau, Lithium market research – global supply, future demand and price development, Energy Storage Mater., 2017, 6, 171–179 CrossRef.
- I. Ore, I. O. Pigments, P. Rock, Q. Crystal, R. Earths and S. Ash, Mineral Commodity Summaries 2021, 2021 Search PubMed.
- S. V. Demin, N. A. Shokurova, L. I. Demina, L. G. Kuz’mina, V. I. Zhilov and A. Y. Tsivadze, Effects of Side-Chain Substituents in Benzo-15-Crown-5 on Lithium Extraction, Russ. J. Inorg. Chem., 2018, 63, 121–127 CrossRef CAS.
- K. Wilcox and G. E. Pacey, Chromogenic benzo- and monoaza-12-crown-4, 13-crown-4 and 14-crown-4 lithium-selective, Talanta, 1991, 38, 1315–1324 CrossRef CAS PubMed.
- C. Shi, Y. Jia, J. Xiao, X. Wang, Y. Yao and Y. Jing, Lithium isotope separation by liquid-liquid extraction using ionic liquid system containing dibenzo-14-crown-4, J. Mol. Liq., 2016, 224, 662–667 CrossRef CAS.
- K. Nishizawa, S. I. Ishino, H. Watanabe and M. Shinagawa, Lithium isotope separation by liquid-liquid extraction using benzo-15-crown-5, J. Nucl. Sci. Technol., 1984, 21, 694–701 CrossRef CAS.
- N. Alizadeh, A comparison of complexation of Li+ ion with macrocyclic ligands 15-crown-5 and 12-crown-4 in binary nitromethane-acetonitrile mixtures by using lithium-7 NMR technique and ab initio calculation, Spectrochim. Acta, Part A, 2011, 78, 488–493 CrossRef PubMed.
- A. Pálsdóttir, C. A. Alabi and J. W. Tester, Characterization of 14-Crown-4 Ethers for the Extraction of Lithium from Natural Brines: Synthesis, Solubility Measurements in Supercritical Carbon Dioxide, and Thermodynamic Modeling, Ind. Eng. Chem. Res., 2021, 60, 7926–7934 CrossRef.
- Z. Zhang, Y. Jia, B. Liu, H. Sun, Y. Jing, Q. Zhang, F. Shao, M. Qi and Y. Yao, Study on behavior of lithium ion in solvent extraction and isotope separation, J. Mol. Liq., 2021, 324, 114709 CrossRef CAS.
- H. Wang, J. He, J. Liu, S. Qi, M. Wu, J. Wen, Y. Chen, Y. Feng and J. Ma, Electrolytes Enriched by Crown Ethers for Lithium Metal Batteries, Adv. Funct. Mater., 2021, 31, 1–9 Search PubMed.
- Y. Oba, M. Okuhata, T. Osakai and T. Mochida, Solvate and protic ionic liquids from aza-crown ethers: Synthesis, thermal properties, and LCST behavior, Phys. Chem. Chem. Phys., 2018, 20, 3118–3127 RSC.
- D. Taziaux, J. P. Soumillion and J. L. Habib Jiwan, Photophysical and complexing properties of new fluoroionophores based on coumarin 343 linked to rigidified crown-ethers, J. Photochem. Photobiol., A, 2004, 162, 599–607 CrossRef CAS.
- S. Obst and H. Bradaczek, Molecular dynamics study of the structure and dynamics of the hydration shell of alkaline and alkaline-earth metal cations, J. Phys. Chem., 1996, 100, 15677–15687 CrossRef CAS.
- A. Williamson, XXII. – On Etherification, J. Chem. Soc., 1852, 4, 229–239 Search PubMed.
- T. Laue and A. Plagens, Namen- und Schlagwort-Reaktionen der Organischen Chemie, Teubner, 4th edn, 2004 Search PubMed.
- W. Bin Huang, Y. Guo, J. A. Jiang, X. D. Pan, D. H. Liao and Y. F. Ji, An efficient strategy for protecting dihydroxyl groups of catechols, Synlett, 2013, 741–746 Search PubMed.
- J. W. Yuan, H. Y. Qiu, P. F. Wang, J. A. Makawana, Y. A. Yang, F. Zhang, Y. Yin, J. Lin, Z. C. Wang and H. L. Zhu, Synthesis of caffeic acid amides bearing 2,3,4,5-tetrahydrobenzo[ b][1,4]dioxocine moieties and their biological evaluation as antitumor agents, Molecules, 2014, 19, 7269–7286 CrossRef PubMed.
- A. Bey, O. Dreyer and V. Abetz, Thermodynamic analysis of alkali metal complex formation of polymer-bonded crown ether, Phys. Chem. Chem. Phys., 2017, 19, 15924–15932 RSC.
- P. W. Linder and K. Murray, Correction of formation constants for ionic strength, from only one or two data points: An examination of the use of the extended Debye-Hückel equation, Talanta, 1982, 29, 377–382 CrossRef CAS PubMed.
- G. Donnay and J. W. Gryder, The oxygen coordinations of lithium, J. Chem. Educ., 1965, 42, 223 CrossRef CAS.
- C. W. Bock, A. Kaufman and J. P. Glusker, Coordination of Water to Magnesium Cations, Inorg. Chem., 1994, 33, 419–427 CrossRef CAS.
- A. F. Holleman and N. E. Wiberg, Lehrbuch der Anorganischen Chemie, Gruyter, Walter de, 102nd edn, 2007 Search PubMed.
- R. G. Parr and R. G. Pearson, Absolute Hardness: Companion Parameter to Absolute Electronegativity, J. Am. Chem. Soc., 1983, 105, 7512–7516 CrossRef CAS.
- E. C. Koch, Acid-base interactions in energetic materials: I. The Hard and Soft Acids and Bases (HSAB) principle - Insights to reactivity and sensitivity of energetic materials, Propellants, Explos. Pyrotech., 2005, 30, 5–16 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cp01076c |
| This journal is © the Owner Societies 2022 |