Open Access Article
Open Access ArticleAdsorption of NO, NO2 and H2O in divalent cation faujasite type zeolites: a density functional theory screening approach
Ayoub
Daouli
*ab,
Etienne Paul
Hessou
 a,
Hubert
Monnier
c,
Marie-Antoinette
Dziurla
a,
Hubert
Monnier
c,
Marie-Antoinette
Dziurla
 d,
Abdellatif
Hasnaoui
d,
Abdellatif
Hasnaoui
 b,
Guillaume
Maurin
b,
Guillaume
Maurin
 e and
Michael
Badawi
e and
Michael
Badawi
 *ad
*ad
aLaboratoire de Physique et Chimie Théoriques, CNRS, Université de Lorraine, Vandœuvre-lès-Nancy, France. E-mail: ayoub.daouli@univ-lorraine.fr; michael.badawi@univ-lorraine.fr
bLS2ME – Polydisciplinary Faculty of Khouribga –Sultan Moulay Slimane University of Beni Mellal, Khouribga, Morocco
cINRS Institut National de Recherche et de Sécurité, Vandœuvre-lès-Nancy, France
dIUT de Moselle-Est, Université de Lorraine, Saint-Avold, France
eICGM, Université de Montpellier, CNRS, ENSCM, Montpellier, France
First published on 3rd June 2022
Abstract
Emissions of diesel exhaust gas in confined work environments are a major health and safety concern, because of exposition to nitrogen oxides (NOx). Removal of these pollutants from exhaust gas calls for engineering of an optimum sorbent for the selective trapping of NO and NO2 in the presence of water. To this end, periodic density functional theory calculations along with a recent dispersion correction scheme, namely the Tkatchenko–Scheffler scheme coupled with iterative Hirshfeld partitioning TS/HI, were performed to investigate the interactions between NO, NO2, H2O and a series of divalent cation (Be2+, Mg2+, Ca2+, Sr2+, Ba2+, Fe2+, Cu2+, Zn2+, Pd2+, and Pt2+) faujasites. This enabled the identification of the optimum zeolites to selectively capture NOx in the presence of H2O, with respect to two important criteria, such as thermodynamic affinity and regeneration. Our results revealed that Pt2+ and Pd2+ containing faujasites are the best candidates for effective capture of both NO and NO2 molecules, which paves the way towards the use of these sorbents to address this challenging application.
1. Introduction
Air quality is currently a major environmental concern. The concentration of pollutants, such as carbon oxides (CO and CO2), ozone (O3), volatile organic compounds (VOCs), nitrogen monoxide (NO) and nitrogen dioxide (NO2), released by thermal motors needs to be reduced,1,2 since these chemicals are known to be highly toxic and responsible for serious health problems, e.g. chronic obstructive pulmonary disorder, respiratory infections and lung cancer among others.2 According to the World Health Organization (WHO), 90% of the world population breathe polluted air, and seven million people die each year from diseases caused by air pollution.3 Since humans spend more than 80% of their life indoors, including living and working places where pollutant levels are estimated to be typically 5 to 10 times higher than outdoor, they are particularly exposed to the typical indoor pollutants including NO, NO2, CO and VOCs.4Along with ozone (O3), NOx (both NO and NO2) are amongst the most harmful indoor air pollutants.5 NO gas is a colorless and odorless gas, while NO2 is a reddish-brown gas with a strong smelly odor, and its toxicity is five times higher than that of NO.6,7
Moreover, NOx released by engines (300–1000 ppm for both NO and NO2) in a confined work environment lacking ventilation and exhaust treatment represents a major health and safety problem.8,9 In France, almost 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 workers are exposed to such emissions. As a result, European Union regulatory requirements have attempted to address these concerns through EU Directive 2017/164, which set an occupational exposure level limit value for NO and NO2 emitted from engines in a working environment of 2 and 0.5 ppm, respectively.10 Therefore, the reduction of NOx emissions in exhaust gases of off-road vehicles is a current priority in occupational risk prevention. This calls for the development of efficient technologies to comply with these health standards and for improving health and safety in confined work environments.
000 workers are exposed to such emissions. As a result, European Union regulatory requirements have attempted to address these concerns through EU Directive 2017/164, which set an occupational exposure level limit value for NO and NO2 emitted from engines in a working environment of 2 and 0.5 ppm, respectively.10 Therefore, the reduction of NOx emissions in exhaust gases of off-road vehicles is a current priority in occupational risk prevention. This calls for the development of efficient technologies to comply with these health standards and for improving health and safety in confined work environments.
Several strategies such as NOx storage-reduction (NSR) and selective catalytic reduction (SCR) have been proposed in the last decade, in order to limit NOx release in lean-burn engines.11–13 Being efficient at high temperatures, they have already been implemented in light vehicles where the engine temperature quickly increases thanks to their continuous run. However, these techniques face several problems.14 In particular, their efficiency for NOx reduction becomes limited in construction site machinery running discontinuously and therefore subjected to several cold starts, leading to low exhaust temperature.15
Therefore, an effective sorbent for the selective capture of low concentrations of NO and NO2 in the presence of water 16 needs to be engineered by paying attention that residual H2O can react with NO2 for producing NO and nitric acid.16,17 In the context of ecological transition, adsorbent regeneration also appears crucial. Thus, a subtle balance between high selectivity and easy regeneration is required.
Recent studies revealed that inorganic sorbents such as zeolites can address the capture of NOx.17,18 Indeed, on the basis of 200 existing zeolites,19–21 mordenite (MOR),22,23 MFI (ZSM5),24,25 and faujasite (FAU) architectures have been proposed as promising candidates for NOx removal.17,26 However, the identification of the most efficient zeolite in terms of topology, nature and concentration of extra-framework cations requires many experiments. To circumvent this limitation, molecular modeling is a powerful tool to narrow down the list of potential adsorbents.27,28 Specifically, molecular simulations can assess accurately the adsorption energies and capacities for a large number of sorbents/sorbate pairs and therefore becomes a reliable screening tool for identifying the most efficient sorbents for the selective trapping of a target sorbate.
Herein, we present a screening approach based on density functional theory (DFT) calculations to assess the efficiency and the selectivity of zeolite materials for the target applications.29–32 Typically, faujasites are good candidates for NOx trapping, thanks to the presence of the extra framework cations that can act as strong adsorption sites combined with their large supercages that enable to optimize the amount adsorbed.13,17,33,34 Furthermore, the structure composition (Si/Al ratio) can be fine-tuned from one up to infinite35 by modifying the hydrothermal synthesis (ratios 1 to 3) or by applying post-synthetic modifications (ratio 3 onwards).
Among this family of faujasites, the Y version36 was first synthesized with a Si/Al ratio of about 2.5, exhibiting a good chemical/thermal stability and high cation exchange capacity, and therefore became industrially widely used as a molecular sieve and an adsorbent.37,38 In addition, the Si/Al ratio of Y zeolites can be further increased and adjusted through stream and/or acid treatments,35 yet still maintaining crystallinity. As a result, a zeolite featuring improved hydrothermal stability “Ultra Stable Y” (USY) was conceived and labeled for all structures with a Si/Al ratio of about 6 and above. Y and USY zeolites have shown remarkable promise in many industrial applications.39–41
More specifically, the objectives of the present work are to investigate the preferential adsorption configurations of NO, NO2 and H2O in a series of divalent cation faujasite Y and to assess the associated interaction energies and the host/guest charge transfer. To this purpose, our periodic DFT investigations focused on a series of ten divalent cation faujasite Y (Be2+, Mg2+, Ca2+, Sr2+, Ba2+, Zn2+, Cu2+, Fe2+, Pd2+ and Pt2+) with a model Si/Al ratio equal to 23. The optimal divalent cation Pt2+ was further incorporated in the common zeolite Y (Si/Al = 2.4) and its adsorption behavior with respect to the 3 molecules mentioned above was predicted to confirm the potential of this zeolite for a selective capture of NOx. The bond activation upon adsorption of the studied molecules was later analyzed to gain insight into the regeneration of these materials.
2. Computational and structural details
2.1 Structural model
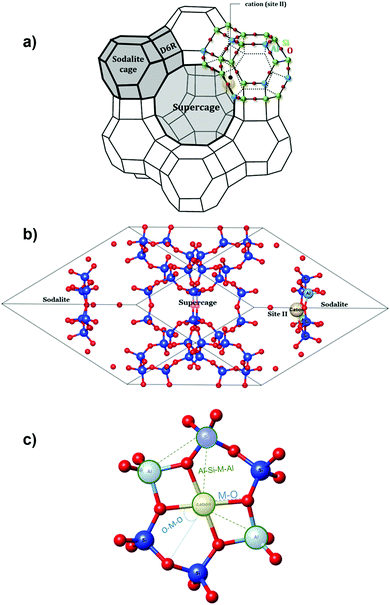
Faujasite is a three-dimensional network belonging to the wide-pored zeolites (Fig. 1a). Its structure consists of 6.6 Å diameter sodalite cages (β cages) connected through hexagonal prisms (D6R) with an opening of 2.3 Å. By other windows, these β cages are connected to 12.4 Å diameter supercages (α cages) which are also linked together by a 7.4 Å diameter hexagonal window (12MR) leading to the formation of a porous network.42The purely siliceous structure of faujasite belongs to the Fd3m symmetry space group,43 with the standard cubic lattice containing 576 atoms (Si192O384) and characterized by a lattice constant of 25.028 Å.44,45 In the present study, to minimize computational efforts, only a 144 atom rhombohedral primitive cell was considered containing 8 hexagonal windows linking the two supercages to the sodalite cages and characterized by the following cell dimensions: a = b = c = 17.3432 Å, α = β = γ = 60° and V = 3688.68 Å3.
To obtain a Si/Al ratio equal to 23, two Si atoms were exchanged by two Al atoms in the primitive cell (Fig. 1c). Therefore, the molecular formula of the investigated cell is M1Al2Si46O96, with M = Be2+, Mg2+, Ca2+, Sr2+, Ba2+, Zn2+, Sn2+, Fe2+, Pt2+ or Pd2+. In zeolites, the Loewenstein rule which governs Al–O interactions excludes the sharing of one oxygen atom by two AlO4 tetrahedra.46 This means that the Al–O–Al bond formation is excluded from the zeolite framework and that the distance between two aluminum cations in a 6MR (SiO2/Al2O3) ring needs to be as large as possible (Fig. 1c).44
Several crystallographic investigations have shown that for a Si/Al ratio greater than five, site II (Fig. 1) is the most favorable to be occupied by cations.47,48 This site is situated within the supercage, more particularly upon the hexagonal window connecting the sodalite cage to the supercage. In addition, this site is known to be accessible to most of the adsorbates.49,50 Therefore, in our study, only site II was considered for the location of the different envisaged divalent cations, consistent with previous studies.51,52
2.2 DFT adsorption calculations
The adsorption modes and energies of NOx and H2O on site II of exchanged cationic zeolites were studied through periodic DFT calculations53,54 using the code VASP (Vienna Ab Initio Simulation).55 The functional of Perdew, Burke, Ernzerhof (PBE) of the generalized gradient approximation was applied (GGA).56 The electron–ion interactions were described using the projector augmented plane wave (PAW) method developed by Blöchl57 and adjusted later by Kresse and Joubert.58 The plane wave cutoff energy was set to 450 eV and the Brillouin zone sampling at the Γ-point. The Kohn–Sham self-consistent total energy differences were converged within 10−6 eV.54 A 0.1 eV Gaussian smearing was applied and the atomic positions were fully relaxed until all forces were below 0.02 eV Å−1 per atom.59To describe the adsorption of the different guest molecules in the faujasite with a high accuracy, van der Waals (vdW) interactions have to be accounted for.60–63 For this, the Tkatchenko–Scheffler scheme with iterative Hirshfeld partitioning (TS/HI) was considered. This vdW correction method was demonstrated to accurately describe the dispersion interactions of small molecules over exchange zeolites.64
Spin-polarized (collinear) calculations were performed for all the cations. Fe2+ with a 3d6 orbital was found to be 1.74 eV more stable in the high spin state compared to the low spin state. To further treat the strong correlation effects of Fe, the GGA + U method of Hubbard65 was applied with an effective Hubbard parameter Ueff of 4.0.
The interaction energies between the guest molecules and the cation containing faujasites were calculated at 0 K using the following equation:
| ΔEint = E(FAU+guest) − (EFAU + Eguest) | (1) |
In a similar procedure, the dispersion force contribution ΔEdisp to the interaction energy is given by
| ΔEdisp = Edisp(FAU+guest) − Edisp(FAU) − Edisp(guest) | (2) |
In order to further improve our understanding and to look at the effect of the interaction of the adsorbed molecule with the zeolite, both the charge density difference (Δρ) and Bader charge difference (ΔQ) were determined.66–68
The charge density difference can be calculated taking the superposition (obtained from the initial condition of the self-consistency cycle) of non-interacting atoms (or isolated) as the reference. The visualization of the charge density difference (Δρ) upon the adsorption of NOx and H2O into exchanged FAU requires three calculations as shown in the equation below:
| Δρ = ρFAU+guest − ρFAU − ρ+guest | (3) |
On the other side, the Bader approach consists of exploiting the topological properties of the charge density to partition the space in several regions, and the boundary of each Bader volume is defined as the surface through which the charge density gradient has a zero flux. Thus, the partial charge and polarization of single atoms are determined. To obtain the difference in an electronic charge ΔQ, the following equation was used:
| ΔQ = QFAU-guest − (QFAU + Qguest) | (4) |
3. Results and discussion
3.1. Geometry of the cation exchanged FAU zeolites
The locations of all tested cations (M = alkali metals and transition metals) in the empty and guest-loaded faujasites were determined by calculating different geometric parameters such as M–O distances (determined as the mean distance between M and the nearest 6MR oxygens), O–M–O angles (determined as the angle between the cation and two neighboring oxygens) and Al–Si–M–Al dihedral angles between M and the 6MR window plane (Fig. 1c). All distances and angles values are listed in Tables 1 and 2, respectively.| Cations | Be2+ | Mg2+ | Ca2+ | Sr2+ | Ba2+ | Zn2+ | Cu2+ | Fe2+ | Pd2+ | Pt2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| Ionic Radii (pm) | 45 | 72 | 100 | 118 | 135 | 74 | 73 | 78 | 86 | 80 |
| Bare zeolite | 1.66 | 2.06 | 2.35 | 2.53 | 2.70 | 1.97 | 2.03 | 2.19 | 2.07 | 2.06 |
| NO | 1.69 | 2.10 | 2.39 | 2.53 | 2.71 | 2.00 | 2.05 | 2.09 | 2.22 | 2.24 |
| NO2 | 1.70 | 2.10 | 2.40 | 2.54 | 2.71 | 1.99 | 2.05 | 2.23 | 2.07 | 2.07 |
| H2O | 1.73 | 2.12 | 2.41 | 2.55 | 2.73 | 2.04 | 2.05 | 2.08 | 2.07 | 2.07 |
| Cations | Be2+ | Mg2+ | Ca2+ | Sr2+ | Ba2+ | Zn2+ | Cu2+ | Fe2+ | Pd2+ | Pt2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| Ionic Radii (pm) | 45 | 72 | 100 | 118 | 135 | 74 | 73 | 78 | 86 | 80 |
| Bare zeolite | 63.8 | 74.0 | 67.2 | 63.2 | 59.7 | 66.8 | 77.1 | 72.0 | 75.0 | 74.5 |
| NO | 65.0 | 72.9 | 67.0 | 63.1 | 59.3 | 67.4 | 67.3 | 69.1 | 71.0 | 70.4 |
| NO2 | 64.7 | 73.0 | 66.9 | 63.0 | 59.6 | 67.6 | 76.9 | 70.0 | 74.8 | 74.4 |
| H2O | 67.4 | 72.3 | 66.5 | 62.8 | 59.5 | 65.5 | 76.8 | 71.4 | 75.1 | 74.6 |
 | ||
| Fig. 2 An illustration of how the cation size influences its initial position regarding the 6MR windows plane: (a) alkaline earth family and (b) transition metal series. | ||
Furthermore, upon increasing the cation radius from Be2+ to Ba2+, we noticed an increase in the M–O distance from 1.64 Å to 2.70 Å, respectively. Moreover, a decrease in the O–M–O angle from 74.0° to 59.7° can be observed when switching from magnesium to barium. This result is in agreement with a recent work which showed that more the cations are out of the zeolite windows plane, lower are the values of the O–M–O angle.69 Variations of these three geometrical parameters, in line with the ionic radius of the cation reported in Table 1, indicate that the cation position affects the interactions with the adsorbates.
Similarly, to alkaline earth metals, the relative positions of metal cations (Zn2+, Cu2+, Fe2+, Pd2+ and Pt2+) within the zeolite structure were also described by the determination of the M–O distances and O–M–O angles (Tables 1 and 2). In addition, the calculations of the dihedral angles revealed values ranging from 2.5° to 5.5° which are illustrated in Fig. 2. These results clearly confirm that the most planar geometry between M and the 6MR window is obtained with Zn2+ and contradicts the fact that the cation position in the 6MR windows plane varies in the same way as its ionic radius. Similar trends were previously observed using the ZSM-5 zeolite structure.70
 | ||
| Fig. 3 Illustration of the cation shift upon NO adsorption on the alkaline earth, calcium (a) and transition metal, platine (b) zeolites. | ||
Determination of the O–M–O angles (Table 2) for alkaline earths showed that Be2+ is the most adsorption-sensitive cation, as its corresponding angle increased from 3.6°, 1.2° and 1° after adsorption of H2O, NO and NO2, respectively.
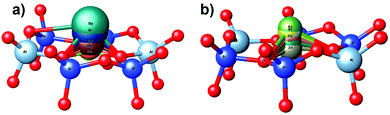
Similar trends were also observed for transition metal exchanged zeolites. Zn2+ was found to be sensitive to water as the O–M bond slightly increased from 1.95 Å to 2.04 Å (Table 1). Pt and Pd cations were found to be affected by NO adsorption as their O–M bonds significantly increased by 0.18 and 0.15 Å, respectively. In contrast to alkaline earth metals, for transition metals, the O–M–O angle decreases as the O–M bond increases, which could be due to the cation shift towards the side of the 6MR windows (near the Al atom) (Fig. 3b), while, with alkaline-earth metals, the cations remain in the 6MR windows plane (Fig. 3a).
These different geometric features of the two categories of cation–faujasites suggest distinct adsorption behavior and an associated interaction energy.
3.2. Energetics and iso-surface electron density
To investigate the ability of the cation exchanged FAUs to capture NOx, several adsorption modes were considered, and the most stable ones were selected (Fig. 4). Calculated energies ΔEint (kJ mol−1) together with their associated dispersion contribution ΔEdisp (kJ mol−1) are reported in Table 3. | ||
| Fig. 4 Different adsorption modes for (a) NO, (b) NO2 and (c) H2O in FAU exchanged with divalent cations. | ||
| Be2+ | Mg2+ | Ca2+ | Sr2+ | Ba2+ | Zn2+ | Cu2+ | Fe2+ | Pd2+ | Pt2+ | |
|---|---|---|---|---|---|---|---|---|---|---|
| NO | −37.6 (−11.8) | −44.5 (−10.2) | −39.0 (−10.9) | −35.0 (−11.1) | −26.6 (−10.7) | −48.7 (−10.3) | −107.8 (−9.9) | −155.5 (−9.1) | −168.7 (−16.3) | −145.6 (−15.9) |
| NO2 | −33.8 (−16.6) | −53.2 (−12.7) | −49.9 (−13.6) | −42.4 (−12.5) | −21.8 (−12.9) | −42.1 (−13.3) | −27.2 (−14.4) | −53.7 (−14.2) | −74.2 (−13.0) | −84.7 (−15.4) |
| H2O | −114.8 (−10.8) | −112.0 (−7.7) | −98.9 (−8.6) | −88.7 (−8.6) | −74.6 (−7.3) | −105.3 (−9.1) | −81.6 (−8.8) | −56.3 (−9.4) | −48.2 (−19.0) | −33.2 (−16.7) |
Fig. 4 shows that NO is adsorbed through its nitrogen atom, whereas NO2 displays three different adsorption modes depending on whether it interacts through nitrogen or through one or two oxygen atoms. Moreover, water adsorbs preferentially through its oxygen atom although an additional interaction can occur with one of its hydrogen atoms.
3.2.1.1 Alkaline earth metals Be2+, Mg2+, Ca2+, Sr2+, and Ba2+. Among the three adsorbates, NO shows the weakest affinity for the alkaline earth metal-exchanged faujasites with a calculated interaction energy for beryllium predicted to be −37.6 kJ mol−1, including −11.8 kJ mol−1 vdW contribution, i.e. 31% of the total interaction energy (Table 3). Furthermore, the NO interaction energy decreases progressively from −44.5 kJ mol−1 with Mg2+ to −26.6 kJ mol−1 with Ba2+, including an average of −10 kJ mol−1 vdW contribution, i.e. 27%. These low values indicate a decrease in the interaction force between NO and the alkaline earth series, which is correlated with an increase in the ionic radius of this group of metals. Such results indicate that increasing the electropositivity of the charge-compensating cation leads to weaker adsorption.
3.2.1.2 Transition metals Zn2+, Fe2+, Cu2+, Pd2+ and Pt2+. The interaction energy of NO significantly increases from Zn2+ exchanged faujasite to Pt2+ exchanged faujasite (Table 3). The values of the Zn2+ exchanged faujasites are close to those observed for faujasites exchanged with alkaline earth metals. Higher interaction energies, such as −107.8 kJ mol−1, −155.5 kJ mol−1, −168.7 kJ mol−1 and −145.6 kJ mol−1, have been calculated for Cu2+, Fe2+, Pd2+ and Pt2+, respectively. The corresponding dispersion contributions are −9.9 kJ mol−1, −9.1 kJ mol−1, −16.3 kJ mol−1 and −15.9 kJ mol−1, respectively, representing an average of only 7% of the total interaction energy. These results are in line with those of Kanougi et al.71 who computed an interaction energy of −112.6 kJ mol−1 for NO in ZSM-5 exchanged with Pd2+.
Similar findings were also reported by Wang et al.72 and Sun et al.73 for the adsorption of NO and N2O on Fe-BEA and H-BEA zeolites on the one hand, and NO and CO on Fe/ZSM-5 on the other hand. Wang et al. revealed that Fe(III) exchanged BEAs show the highest affinity for NO with an interaction energy of −123 kJ mol−1, very close to the value we found for Fe(II)–FAU. However, Sun et al. obtained a much higher energy of −258 kJ mol−1 for the ZSM-5 exchanged with Fe(II) most probably due to the location of the Fe(II) on the 8MR site. Many studies reported that cations located in the 8MR sites lead to higher interaction energies compared to the cations located in the 6MR site.74,75 Furthermore, these previous studies revealed that, in all investigated zeolites, NO molecules preferentially adsorb via their nitrogen rather than their oxygens, in agreement with our findings reported in Fig. 4.
Fig. 5 represents the negative and positive iso-surfaces between Ba–NO and Pt–NO. The high electrostatic interactions between nitrogen and Pt2+ were confirmed through the high charge transfer, together with the Bader method based computed ΔQ (0.14 e; −0.17 e) for Pt and N, respectively. It is not the case with the Ba cation where its Bader charge was almost unaffected by the adsorption of NO. The calculated distance between the Pt atom and N atom of NO (dPt-N =1.92 Å) confirmed this result, indicating a chemical bond formation between the cations and the nitrogen atom of NO, with the formation of complexes.76,77
3.2.2.1 Alkaline earth metals Be2+, Mg2+, Ca2+, Sr2+, and Ba2+. The NO2 interaction energy varies between −53.2 kJ mol−1 and −21.8 kJ mol−1 from Mg2+ to Ba2+ (Table 3). This relatively weak interaction is in line with the absence of the charge transfer between Ba2+ and NO2 (Fig. 6a). The significant decrease of the interaction energy along this family of cations follows the decrease in the hardness of the cation, leading to a weaker interaction with hard base molecules, i.e. HSAB theory (Hard Soft Bases and Acids) introduced by Parr Pearson.78
3.2.2.2 Transition metals Zn2+, Fe2+, Cu2+, Pd2+ and Pt2+. For faujasites exchanged with transitions metals, Table 3 shows that NO2 is less strongly adsorbed as compared with NO and preferentially interacts through its oxygen atoms for most of the cations as shown in Fig. 4b. The total interaction energy reaches a maximum of −53.7 kJ mol−1 for Fe2+, which is similar to the value observed for Mg2+ (−53.2 kJ mol−1), reflecting the same interaction mode of NO2 with these two cations. Zinc and copper show the lowest interaction values of −42 and −27 kJ mol−1 with NO2, respectively.
Benco79 used DFT to study the adsorption of a set of diatomic and triatomic probe molecules on [Zn–Zn]2+ particles in a ferrierite zeolite. NO and H2O molecules were found to interact with a single Zn atom, resulting in interaction energies of −48.8 and −100 kJ mol−1, respectively, very similar to our findings (Table 3). However, the NO2 molecule was found to interact with both Zn atoms of the [Zn–Zn]2+ particle, indicating the 54 kJ mol−1 higher adsorption as compared to our results.
Pt2+ and Pd2+ are the only cations that interact with NO2 through its nitrogen atom and, thus again, they revealed the highest adsorption values of −84.7 and −74.2 kJ mol−1, respectively. The corresponding vdW contribution was estimated to −15.4 kJ mol−1 for Pt2+. The strong interaction of NO2 with the Pt cation was confirmed by the iso-surface electron density presented in Fig. 6b.
3.2.3.1 Alkaline earth metals Be2+, Mg2+, Ca2+, Sr2+, and Ba2+. As shown by their high interaction energy with water around −98 kJ mol−1 (Table 3), all studied cations display a high affinity for this guest, with a vdW contribution of 9%. According to the HSAB theory,78 alkaline earth metals are considered as hard acids, showing a high affinity for hard bases such as H2O, which is confirmed by the iso-surface electron density (Fig. 7a). These results are in accordance with the theoretical work of Benco and Tunega69 on mordenite.
3.2.3.2 Transition metals Zn2+, Fe2+, Cu2+, Pd2+ and Pt2+. With interaction values of −105.3 kJ mol−1 and −81.6 kJ mol−1, Zn2+ and Cu2+ display the strongest interactions with water (Table 3). These values are close to those calculated for alkaline earth metals (Table 3). Considering the HSAB theory, Zn2+ and Cu2+ are the hardest acids amongst all transition metals tested.
On the other hand, Pd2+ and Pt2+ exhibit weak interactions with water as indicated by the low interaction energies observed, of −48.2 kJ mol−1 and −33.2 kJ mol−1, respectively. Indeed, located below Zn2+ and Cu2+ in the periodic table, Pd2+ and Pt2+ are softer cations. The iso-surface electron-density representation (Fig. 7b) confirms the weak interaction between Pt and water.
Recently, Mandal et al.80 provided insights into the molecular structure of Pd cations in SSZ-13 zeolites and their interaction with H2O and NO using experimental and computational analyses. The most stable position of Pd was found to be equal to the site II used here (Fig. 1), in which the six-membered ring has two aluminum atoms on opposite sides. Once again, the interaction energies of −42 and −123 kJ mol−1 for H2O and NO2 molecules, respectively, agree with the values listed in Table 3.
3.3. Selection of the most effective cation to selectively capture NOx
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm),81 the adsorbent will have to show a much higher affinity for NO and NO2 than for water.
000 ppm),81 the adsorbent will have to show a much higher affinity for NO and NO2 than for water.
 | ||
| Fig. 8 Total interaction energies of NO, NO2, and H2O with divalent cation-exchanged faujasites, calculated using the PBE + TS/HI level of theory. | ||
Be2+, Mg2+, Ca2+, Sr2+, Ba2+ Zn2+, and Cu2+-exchanged FAUs exhibit a higher or equivalent affinity for water than that for NOx (Fig. 8). NO and NO2 have almost the same interaction energy on a given cation, and their interaction decreases in absolute value from Mg2+ (49 kJ mol−1) to Ba2+ (23.5 kJ mol−1). This indicates that these materials will be poor sorbents for NOx and unsuitable for the intended pollution control application.
As shown in Fig. 8, Fe2+-exchanged FAU exhibits a similar affinity for water and NO2 (−56.3 and −53.7 kJ mol−1, respectively), but much higher affinity for NO (−155 kJ mol−1). This means that water is expected to play a detrimental role on NO2 adsorption. However, the NO2 molecule appears to be highly reactive with water in zeolites, leading to NO formation;82 therefore, Fe2+ could be an appropriate cation for selectively trapping NOx in the presence of water owing to its high affinity for NO.
Palladium and platinum are the only cations showing a higher affinity for NO and NO2 compared to water (Fig. 8 and Table 3). NO shows a high affinity for both cations (−145.6 and −168.7 kJ mol−1, respectively) followed by NO2 (−84.7 and −74.2 kJ mol−1). These interaction energies are significantly higher than those of water (−33.2 and −48.2 kJ mol−1 for Pd2+ and Pt2+, respectively). Therefore, water is not expected to inhibit the adsorption of NOx in these zeolites. These results reveal that in terms of thermodynamic selectivity, Pd2+, Pt2+ and in a lesser extend Fe2+ forms are clearly the most interesting faujasites for the selective capture of NOx from water.
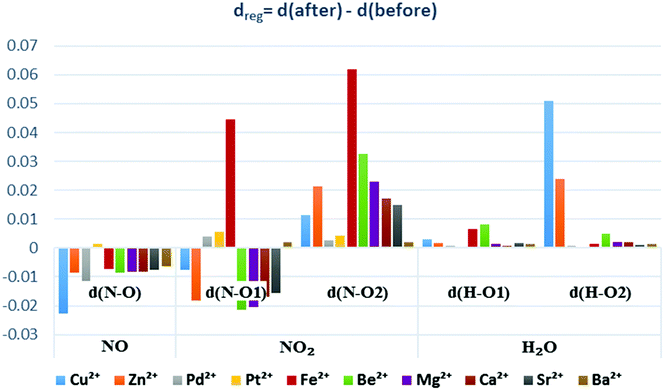
For alkaline earth cations, the bond length analysis for the pristine and loaded faujasites (Fig. 9) revealed that the NO bond length slightly decreases (from 0.006 to 0.008 Å for most cations). The “N–O1” bond length with the first oxygen atom in NO2 is almost unaffected but the “N–O2” bond with the second oxygen is stretched with an average variation of 0.02 Å. Concerning H2O, the distances of the two water bonds (O–H) are barely changed. In terms of regeneration, alkaline earth cations can safely be used for NO removal. However, NO2 may develop undesirable by-product formation by chemically reacting with these cations and other molecules. One additional observation in Fig. 9 is that beryllium has the most noticeable differences in bond lengths (both stretched and retracted); this may be attributed to its small ionic radius (Table 1). Therefore, we can suggest that the smaller the radius, the higher the probability of activating the bond length becomes.
Looking at transition metals, all five cations can be safely used to trap NO, as their (N–O) bond lengths become shorter than those in the gas phase (Fig. 9). For NO2, the significant activation of both N–O1 and N–O2 bonds occurs for Fe2+, Cu2+ and Zn2+, while only a slight variation in the bond length from 0.004 and 0.005 Å is observed for Pd2+ and Pt2+, respectively. These variations are too small for affecting the regeneration process and are therefore negligible. Concerning water adsorption, a minor variation in the H–O bond length was observed for all cations, except for zinc and copper where the variation in the O–H bond of water was significant (Fig. 9).
Indeed, the analysis of the bond activation shows that only Pd2+ and Pt2+ can be effectively used for NOx trapping. In the following, we will evaluate the potential of Pt2+, which is less costly than Pd2+, when embedded in a realistic faujasite Y structure characterized by a Si/Al ratio of 2.4.
3.4. Evaluation of Pt(II)–NaY for NOx capture
To obtain a Pt(II)–NaY structure (Si/Al ratio = 2.4), 14 Si atoms were substituted by 14 Al atoms in the primitive cell (Fig. 10). The distribution of platinum cations in the zeolitic Y-structure may have an impact on the adsorption mechanisms of NOx. Numerous experimental and, more recently, simulation methods have been reported on the location of cations in the faujasite. Frising and Leflaive47 have gathered and presented in an extensive review, all the different results that have been published in the literature over the years. | ||
| Fig. 10 The Pt–NaY zeolite primitive cell displayed in balls and stick cells, with three accessible sites. Site II (supercage), site I (hexagonal prism) and site I’(sodalite cage). | ||
For platinum, only one reference was found where the Na–Y zeolite was partially Pt-exchanged.85 Therefore, the molecular formula of the investigated cell is Pt4Na6Al14Si34O96. In this cell, unexchanged Na+ cations seem to prefer site II (supercage) followed by site I (at the center of the hexagonal prism), while Pt2+ cations tend to occupy site II and site I’ (in the sodalite cage) (Fig. 10).
 | ||
| Fig. 11 Total interaction energies of NO, NO2, and H2O with platinum exchanged Na–Y zeolites, calculated using the PBE + TS/HI level of theory. | ||
By contrast, the difference in interaction energies of NOx with increasing the cation concentration is surprisingly relatively high, especially since in both cases the adsorption takes place on the same cation position (site II). These observations tend to be driven by the location stability of the cation.
Comparing the [Pt–O] distance between the Pt(II) cation and the D6R oxygens on both Si/Al ratios, it was found that, for the pristine bare zeolite, the platinum cation located on the D6R plane binds strongly to four oxygens, with an average distance of 2.07 Å. Upon adsorption, Pt on zeolite Y was found to be more sensitive to NOx adsorption. The platinum in zeolite Y is displaced from its original position and slightly elevated from the D6R plane with 2.04 Å (Fig. 13e and f) compared to 1.08 Å for USY (Fig. 5).
This higher mobility of the Pt cations in zeolite Y compared to zeolite USY seems to be related to the influence of neighboring cations in the sodalite cage. As shown in Fig. 12, the sodalite cage is strongly perturbed upon NOx adsorption, and the site II Pt cation is pulled out from its original position, subsequently followed by a displacement of the site I' Pt cation towards this vacant site II. This cation confinement effect leads to a greater exposure of the site II Pt cation to the probe molecule compared to the USY zeolite.
The migration of cations from site II to the supercage in zeolite Y has been discussed in many studies: Typically, some of us86 demonstrated by molecular dynamics combined with complex impedance spectroscopy measurements that methanol perturbs the Na–Y zeolite, moving cations from site II to the center of the supercage, followed by hopping of SI’ cations from the sodalite cage into the supercage to fill the vacant SII sites. Klier87 also mentioned that a CO molecule induces the displacement of Cu(I) from less accessible to more accessible positions in Y-type zeolites.
As a result, this leads to a higher interaction energy along with 0.21 and 0.19 |e| charge transfer (eqn (4)) for Pt–NO and Pt–NO2, respectively (Fig. 13e and f), compared to 0.14 and 0.09 |e| (eqn (4)) found previously for the USY zeolite. In contrast, a slight perturbation of the Pt position is observed upon H2O adsorption, demonstrating the low interaction energy and a charge transfer of 0.05 |e| (eqn (4)) (Fig. 13g).
Similarly, the presence of residual sodium from the partially exchanged zeolite may affect our NOx trapping and should therefore be investigated. Adsorption of NOx on these cations revealed very low interaction energies of −24 and −20 kJ mol−1 for NO and NO2, respectively (Fig. 11). While a strong affinity towards water has been achieved at an interaction energy of −55 kJ mol−1 (Fig. 11), in agreement with the HSAB principle.78 These results indicate that the NOx trapping efficiency would be improved in tandem with a high Pt exchange in the Na–Y zeolite.
The reported high NOx interaction energies with Pt cations along with the low interaction energies for Na cations can be understood by studying the differences in the electron density (Δρ) induced by adsorption. In this context, we found that the interactions between N and Pt atoms display a significant electron transfer (Fig. 13e and f), the green density around the NOx molecules reflecting the loss of charges towards the yellow area of the Pt cation. For Na cations, a poor charge transfer can be observed upon adsorption of NOx (Fig. 13a and b), and the water molecule, however, showed a higher charge transfer but mainly in the form of hydrogen bonding with the D6R oxygen atom (Fig. 13c).
For USY (Fig. 4a), the NO molecule was found to be adsorbed by its nitrogen atom on the Y zeolite (Fig. 13e). This adsorption configuration maintained the same bond length, preventing it from activation (Fig. 14), similarly to what has been observed on USY (Fig. 9).
For NO2, due to the large displacement of the cation position, π interactions were observed between the Pt of the Y zeolite and the molecule (Fig. 13f). This adsorption configuration led to a significant stretching of the non-interactive (N–O) bond (Fig. 14). In contrast, for USY, the NO2 molecule was adsorbed only via its nitrogen (Fig. 4b), resulting in no change in its bond length (Fig. 14).
Concerning water, due to the weak hydrogen bonding that occurred during adsorption (Fig. 13g), the bond length of the molecule was negligibly stretched (Fig. 14).
To summarize, the binding analysis shows that Pt(II)–NaY can be safely used for NO removal in the presence of water. However, because of the significant increase of NO2 bond lengths, Pt(II)–NaY appears to be unsuitable for NO2 removal, as it can lead to the formation of undesirable by-products, resulting from the dissociation and the chemical reaction of NO2 with platinum and other molecules. In addition, the revealed high interactions between NOx and Pt may leave the latter difficult to regenerate. Therefore, future studies are needed to investigate the desorption properties of the most promising zeolites to determine the optimal regeneration temperature.
4. Conclusion
In summary, in the context of the prevention of harmful emissions from diesel engines, DFT simulations using the van der Waals correction have been conducted to study the effect of water on the NOx capture by cation containing faujasites. Several divalent cation (alkaline earth metals (Be2+, Mg2+, Ca2+, Sr2+, and Ba2+) and transition metals (Fe2+, Cu2+, Zn2+, Pd2+, and Pt2+)) exchanged faujasites were tested to identify the most selective sorbents toward NO and NO2 contaminants.Overall, when considering thermodynamic selectivity criteria, our interaction energy calculations show that alkaline earth metals, as well as Cu2+ and Zn2+, exhibit a higher affinity for water than for NOx, indicating their unsuitability for the target application, whereas the remaining charge-compensating cations (Fe2+, Pd2+ and Pt2+) behave more efficiently, since all of them show a greater affinity for NO, leaving water with no expected detrimental effect on NOx adsorption, with the exception of Fe2+ that presents a similar affinity for water and NO2.
Nevertheless, the bond activation analysis discarded Fe2+ from the suitable cations list, since a significant activation of the N–O1 and N–O2 bonds is observed for the adsorption of NO2 on Fe2+, resulting in undesired by-product formation, whereby a negligible variation in the bond length is observed for Pd2+ and Pt2+.
The Pt2+cation was then incorporated and studied within the Y zeolite (Si/Al = 2.4). The results highlight that Pt2+ faujasite Y is an interesting material for the removal of NOx from diesel engine exhaust.
This screening method transferable to the adsorption study of other contaminants by zeolite sorbents, including ammonia gas and formaldehyde among others, is expected to guide the experimental effort towards the synthesis of the optimum sorbents to avoid tedious and time-consuming trial-error approach.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was granted access to the HPC resources of TGCC under the allocation 2020 A0080910433 made by GENCI. This work was partially funded by the French National Research Agency NOA Project (ANR-20-CE08-0024). AD, AH and MB acknowledge financial support by the Ministry of Europe and Foreign Affairs, the Ministry of Higher Education, Research and Innovation and the French Institute of Rabat (PHC TOUBKAL 2021 (French-Morocco bilateral program) Grant Number: 12345AB). MB also acknowledges financial support through the COMETE project (COnception in silico de Matériaux pour l’EnvironnemenT et l’Energie) co-funded by the European Union under the program “FEDER-FSE Lorraine et Massif des Vosges 2014–2020”.References
- S. Gligorovski and J. P. D. Abbatt, An indoor chemical cocktail, Science, 2018, 359, 632–633 CrossRef CAS PubMed.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans and International Agency for Research on Cancer, Diesel and gasoline engine exhausts and some nitroarenes., 2014.
- 9 out of 10 people worldwide breathe polluted air, but more countries are taking action, https://www.who.int/news/item/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action.
- W. Jedrychowski, F. Perera, D. Mrozek-Budzyn, E. Mroz, E. Flak, J. D. Spengler, S. Edwards, R. Jacek, I. Kaim and Z. Skolicki, Gender differences in fetal growth of newborns exposed prenatally to airborne fine particulate matter, Environ. Res., 2009, 109, 447–456 CrossRef CAS PubMed.
- European Environment Agency. and European Topic Centre on Air Pollution and Climate Change Mitigation (ETC/ACM)., Air quality in Europe: 2017 report., Publications Office, LU, 2017.
- A. kumar Agrawal, S. K. Singh, S. Sinha and M. K. Shukla, Effect of EGR on the exhaust gas temperature and exhaust opacity in compression ignition engines, Sadhana, 2004, 29, 275–284 CrossRef.
- H. Ahari, M. Smith, M. Zammit, K. Price, J. Jacques, T. Pauly and L. Wang, Impact of SCR Integration on N2O Emissions in Diesel Application, SAE Int. J. Passeng. Cars - Mech. Syst., 2015, 8, 526–530 CrossRef.
- X. Wang, D. Westerdahl, J. Hu, Y. Wu, H. Yin, X. Pan and K. Max Zhang, On-road diesel vehicle emission factors for nitrogen oxides and black carbon in two Chinese cities, Atmos. Environ., 2012, 46, 45–55 CrossRef CAS.
- T. Lee, J. Park, S. Kwon, J. Lee and J. Kim, Variability in operation-based NOx emission factors with different test routes, and its effects on the real-driving emissions of light diesel vehicles, Sci. Total Environ, 2013, 461–462, 377–385 CrossRef CAS PubMed.
- AVIS et RAPPORT de l’Anses relatif à l’Evaluation des méthodes de mesure de 27 substances listées en annexe de la directive (UE) n°2017/164 de la Commission du 31 janvier 2017 | Anses - Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail, https://www.anses.fr/fr/content/avis-et-rapport-de-lanses-relatif-a-levaluation-des-methodes-de-mesure-de-27-substances.
- L. Castoldi, L. Lietti, R. Bonzi, N. Artioli, P. Forzatti, S. Morandi and G. Ghiotti, The NOx Reduction by CO on a Pt−K/Al2O3 Lean NOx Trap Catalyst, J. Phys. Chem. C, 2011, 115, 1277–1286 CrossRef CAS.
- J.-K. Lai and I. E. Wachs, A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5–WO3/TiO2 Catalysts, ACS Catal., 2018, 8, 6537–6551 CrossRef CAS.
- G. Centi and S. Perathoner, Nature of active species in copper-based catalysts and their chemistry of transformation of nitrogen oxides, Appl. Catal., A, 1995, 132, 179–259 CrossRef CAS.
- Z. G. Liu, D. R. Berg, T. A. Swor and J. J. Schauer, Comparative Analysis on the Effects of Diesel Particulate Filter and Selective Catalytic Reduction Systems on a Wide Spectrum of Chemical Species Emissions, Environ. Sci. Technol., 2008, 42, 6080–6085 CrossRef CAS PubMed.
- J. Y. Yan, G.-D. Lei, W. M. H. Sachtler and H. H. Kung, Deactivation of Cu/ZSM-5 Catalysts for Lean NOxReduction: Characterization of Changes of Cu State and Zeolite Support, J. Catal., 1996, 161, 43–54 CrossRef CAS.
- F. Delachaux, C. Vallières, H. Monnier and M.-T. Lecler, Experimental study of NO and NO2 adsorption on a fresh or dried NaY zeolite: influence of the gas composition by breakthrough curves measurements, Adsorption, 2019, 25, 95–103 CrossRef CAS.
- O. Monticelli, R. Loenders, P. A. Jacobs and J. A. Martens, NOx removal from exhaust gas from lean burn internal combustion engines through adsorption on FAU type zeolites cation exchanged with alkali metals and alkaline earth metals, Appl. Catal., B, 1999, 21, 215–220 CrossRef CAS.
- A. Sultana, R. Loenders, O. Monticelli, C. Kirschhock, P. A. Jacobs and J. A. Martens, DeNOx of Exhaust Gas from Lean-Burn Engines through Reversible Adsorption of N2O3 in Alkali Metal Cation Exchanged Faujasite-Type Zeolites, Angew. Chem., Int. Ed., 2000, 39, 2934–2937 CrossRef CAS PubMed.
- IZA Structure Commission, https://www.iza-structure.org/.
- S. Sircar and A. L. Myers, Handbook of Zeolite Science and Technology Chapter22 Gas Separation by Zeolites, CRC Press, 2003 DOI:10.1201/9780203911167.
- P. A. Jacobs, E. M. Flanigen, J. C. Jansen and H. van Bekkum, Introduction to Zeolite Science and Practice, Elsevier Science, 2001 Search PubMed.
- M. Guisnet and J.-P. Gilson, Zeolites for Cleaner Technologies, Catalytic Science Series, Volume 3. Series Edited by Graham J. Hutchings (Cardiff University), Imperial College Press, London, 2002, ISBN 1-86094-329-2, J. Am. Chem. Soc., 2003, 125, 6839 Search PubMed.
- W. M. Meier, The crystal structure of mordenite (ptilolite), Z. Kristallogr. - Cryst. Mater., 1961, 115, 439–450 CrossRef CAS.
- R. J. Argauer and G. R. Landolt, Crystalline zeolite zsm-5 and method of preparing the same, US Pat., US3702886A, 1972 Search PubMed.
- C. Baerlocher, L. B. McCusker and D. H. Olson, Atlas of Zeolite Framework Types, Elsevier, 2007 Search PubMed.
- E. P. Hessou, W. G. Kanhounnon, D. Rocca, H. Monnier, C. Vallières, M. Badawi and S. Lebègue, Adsorption of NO, NO2, CO, H2O and CO2 over isolated monovalent cations in faujasite zeolite: a periodic DFT investigation, Theor. Chem. Acc., 2018, 137, 161 Search PubMed.
- C. R. A. Catlow, B. Smit and R. A. van Santen, Computer Modelling of Microporous Materials, Elsevier, 2004 Search PubMed.
- T. Ayadi, M. Badawi, L. Cantrel and S. Lebègue, Rational approach for an optimized formulation of silver-exchanged zeolites for iodine capture from first-principles calculations, Mol. Syst. Des. Eng., 2022, 7(5), 422–433 RSC.
- M. Chebbi, S. Chibani, J.-F. Paul, L. Cantrel and M. Badawi, Evaluation of volatile iodine trapping in presence of contaminants A periodic DFT study on cation exchanged-faujasite, Microporous Mesoporous Mater., 2017, 239, 111–122 CrossRef CAS.
- G. D. Pirngruber, P. Raybaud, Y. Belmabkhout, J. Čejka and A. Zukal, The role of the extra-framework cations in the adsorption of CO2 on faujasite Y, Phys. Chem. Chem. Phys., 2010, 12, 13534–13546 RSC.
- I. Khalil, H. Jabraoui, S. Lebègue, W. J. Kim, L.-J. Aguilera, K. Thomas, F. Maugé and M. Badawi, Biofuel purification: Coupling experimental and theoretical investigations for efficient separation of phenol from aromatics by zeolites, Chem. Eng. J., 2020, 402, 126264 CrossRef CAS.
- H. Jabraoui, I. Khalil, S. Lebègue and M. Badawi, Ab initio screening of cation-exchanged zeolites for biofuel purification, Mol. Syst. Des. Eng., 2019, 4, 882–892 RSC.
- G. Li, S. C. Larsen and V. H. Grassian, Catalytic reduction of NO2 in nanocrystalline NaY zeolite, J. Mol. Catal. A: Chem., 2005, 227, 25–35 CrossRef CAS.
- J. Szanyi, J. H. Kwak and C. H. F. Peden, The Effect of Water on the Adsorption of NO2 in Na− and Ba−Y, FAU Zeolites:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Combined FTIR and TPD Investigation, J. Phys. Chem. B, 2004, 108, 3746–3753 CrossRef CAS.
A Combined FTIR and TPD Investigation, J. Phys. Chem. B, 2004, 108, 3746–3753 CrossRef CAS. - W. Lutz, Zeolite Y: Synthesis, Modification, and Properties—A Case Revisited, Adv. Mater. Sci. Eng., 2014, 2014, e724248 Search PubMed.
- D. W. Breck, Crystalline zeolite Y, US Pat., US3130007A, 1964.
- E. P. Hessou, H. Jabraoui, I. Khalil, M.-A. Dziurla and M. Badawi, Ab initio screening of zeolite Y formulations for efficient adsorption of thiophene in presence of benzene, Appl. Surf. Sci., 2021, 541, 148515 CrossRef CAS.
- M. U. C. Braga, G. H. Perin, L. H. de Oliveira and P. A. Arroyo, DFT calculations for adsorption of H2S and other natural gas compounds on (Fe, Co, Ni, Cu and Zn)–Y zeolite clusters, Microporous Mesoporous Mater., 2022, 331, 111643 CrossRef CAS.
- A. A. Costa, P. R. S. Braga, J. L. de Macedo, J. A. Dias and S. C. L. Dias, Structural effects of WO3 incorporation on USY zeolite and application to free fatty acids esterification, Microporous Mesoporous Mater., 2012, 147, 142–148 CrossRef.
- L. Cao, F. Xu, Y.-Y. Liang and H.-L. Li, Preparation of the Novel Nanocomposite Co(OH)2/Ultra-Stable Y Zeolite and Its Application as a Supercapacitor with High Energy Density, Adv. Mater., 2004, 16, 1853–1857 CrossRef CAS.
- K. O. Sulaiman, M. Sajid and K. Alhooshani, Application of porous membrane bag enclosed alkaline treated Y-Zeolite for removal of heavy metal ions from water, Microchem. J., 2020, 152, 104289 CrossRef CAS.
- Database of Zeolite Structures, https://www.iza-structure.org/databases/.
- W. H. Baur, On the cation and water positions in faujasite, Am. Mineral., 1964, 49, 697–704 CAS.
- E. Dempsey, G. H. Kuehl and D. H. Olson, Variation of the lattice parameter with aluminum content in synthetic sodium faujasites. Evidence for ordering of the framework ions, J. Phys. Chem., 1969, 73, 387–390 CrossRef CAS.
- F. Porcher, M. Souhassou, Y. Dusausoy and C. Lecomte, The crystal structure of a low-silica dehydrated NaX zeolite, Eur. J. Mineral., 1999, 333–344 CrossRef CAS.
- W. Loewenstein, The distribution of aluminum in the tetrahedra of silicates and aluminates, Am. Mineral., 1954, 39, 92–96 CAS.
- T. Frising and P. Leflaive, Extraframework cation distributions in X and Y faujasite zeolites: A review, Microporous Mesoporous Mater., 2008, 114, 27–63 CrossRef CAS.
- S. Buttefey, A. Boutin, C. Mellot-Draznieks and A. H. Fuchs, A Simple Model for Predicting the Na+ Distribution in Anhydrous NaY and NaX Zeolites, J. Phys. Chem. B, 2001, 105, 9569–9575 CrossRef CAS.
- Z. Nour, D. Berthomieu, Q. Yang and G. Maurin, A Computational Exploration of the CO Adsorption in Cation-Exchanged Faujasites, J. Phys. Chem. C, 2012, 116, 24512–24521 CrossRef CAS.
- H. V. Thang, L. Grajciar, P. Nachtigall, O. Bludský, C. O. Areán, E. Frýdová and R. Bulánek, Adsorption of CO2 in FAU zeolites: Effect of zeolite composition, Catal. Today, 2014, 227, 50–56 CrossRef CAS.
- P. Kumar, C.-Y. Sung, O. Muraza, M. Cococcioni, S. Al Hashimi, A. McCormick and M. Tsapatsis, H2S adsorption by Ag and Cu ion exchanged faujasites, Microporous Mesoporous Mater., 2011, 146, 127–133 CrossRef CAS.
- C.-Y. Sung, S. Al Hashimi, A. McCormick, M. Tsapatsis and M. Cococcioni, Density Functional Theory Study on the Adsorption of H2S and Other Claus Process Tail Gas Components on Copper- and Silver-Exchanged Y Zeolites, J. Phys. Chem. C, 2012, 116, 3561–3575 CrossRef CAS.
- P. Hohenberg and W. Kohn, Inhomogeneous Electron Gas, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Self-Consistent Equations Including Exchange and Correlation Effects, Phys. Rev., 1965, 140, A1133–A1138 CrossRef.
- G. Kresse and J. Hafner, {Ab initio} molecular dynamics for liquid metals, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- S. Chibani, M. Chebbi, S. Lebègue, T. Bučko and M. Badawi, A DFT investigation of the adsorption of iodine compounds and water in H-, Na-, Ag-, and Cu- mordenite, J. Chem. Phys., 2016, 144, 244705 CrossRef PubMed.
- T. Bučko, S. Lebègue, J. G. Ángyán and J. Hafner, Extending the applicability of the Tkatchenko-Scheffler dispersion correction via iterative Hirshfeld partitioning, Chem. Phys., 2014, 141, 034114 Search PubMed.
- T. Bučko, S. Lebègue, J. Hafner and J. G. Ángyán, Improved Density Dependent Correction for the Description of London Dispersion Forces, J. Chem. Theory Comput., 2013, 9, 4293–4299 CrossRef PubMed.
- T. Bučko, S. Lebègue, J. Hafner and J. G. Ángyán, Tkatchenko-Scheffler van der Waals correction method with and without self-consistent screening applied to solids, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 064110 CrossRef.
- A. Tkatchenko and M. Scheffler, Accurate Molecular van der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data, Phys. Rev. Lett., 2009, 102, 073005 CrossRef PubMed.
- A. G. Petukhov, I. I. Mazin, L. Chioncel and A. I. Lichtenstein, Correlated metals and the ${LDA} + U$ method, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 153106 CrossRef.
- W. Tang, E. Sanville and G. Henkelman, A grid-based Bader analysis algorithm without lattice bias, J. Phys.: Condens. Matter, 2009, 21, 084204 CrossRef CAS PubMed.
- E. Sanville, S. D. Kenny, R. Smith and G. Henkelman, Improved grid-based algorithm for Bader charge allocation, J. Comput. Chem., 2007, 28, 899–908 CrossRef CAS PubMed.
- G. Henkelman, A. Arnaldsson and H. Jónsson, A fast and robust algorithm for Bader decomposition of charge density, Comput. Mater. Sci., 2006, 36, 354–360 CrossRef.
- L. Benco and D. Tunega, Adsorption of H2O, NH3 and C6H6 on alkali metal cations in internal surface of mordenite and in external surface of smectite: a DFT study, Phys. Chem. Miner., 2009, 36, 281–290 CrossRef CAS.
- P. Kozyra, J. Załucka, M. Mitoraj, E. Brocławik and J. Datka, From Electron Density Flow Towards Activation: Benzene Interacting with Cu(I) and Ag(I) Sites in ZSM-5. DFT Modeling, Catal. Lett., 2008, 126, 241–246 CrossRef CAS.
- T. Kanougi, H. Tsuruya, Y. Oumi, A. Chatterjee, A. Fahmi, M. Kubo and A. Miyamoto, Density functional calculation on the adsorption of nitrogen oxides and water on ion exchanged ZSM-5, Appl. Surf. Sci., 1998, 130–132, 561–565 CrossRef CAS.
- Y. Wang, Z. Lei, B. Chen, Q. Guo and N. Liu, Adsorption of NO and N2O on Fe-BEA and H-BEA zeolites, Appl. Surf. Sci., 2010, 256, 4042–4047 CrossRef CAS.
- P. Sun, K. Fan, X. Cheng, Z. Qian, Z. Wang, L. Wang and T.-C. Jen, Decoupled NOx adsorption and reduction by CO over catalyst Fe/ZSM-5: A DFT study, Chem. Phys. Lett., 2021, 766, 138344 CrossRef CAS.
- R. Zhang, J. Szanyi, F. Gao and J.-S. McEwen, The interaction of reactants, intermediates and products with Cu ions in Cu-SSZ-13 NH3 SCR catalysts: an energetic and ab initio X-ray absorption modeling study, Catal. Sci. Technol., 2016, 6, 5812–5829 RSC.
- L. A. M. M. Barbosa, R. A. van Santen and J. Hafner, Stability of Zn(II) Cations in Chabazite Studied by Periodical Density Functional Theory, J. Am. Chem. Soc., 2001, 123, 4530–4540 CrossRef CAS PubMed.
- C. Lamberti, A. Zecchina, E. Groppo and S. Bordiga, Probing the surfaces of heterogeneous catalysts by in situ IR spectroscopy, Chem. Soc. Rev., 2010, 39, 4951–5001 RSC.
- K. I. Hadjiivanov and G. N. Vayssilov, Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule, Adv. Catal., Academic Press, 2002, vol. 47, pp. 307–511 Search PubMed.
- R. G. Pearson, Hard and soft acids and bases, HSAB, part 1: Fundamental principles, J. Chem. Educ., 1968, 45, 581 CrossRef CAS.
- L. Benco, Adsorption of small molecules on the [Zn-Zn]2+ linkage in zeolite. A DFT study of ferrierite, Surf. Sci., 2017, 656, 115–125 CrossRef CAS.
- K. Mandal, Y. Gu, K. S. Westendorff, S. Li, J. A. Pihl, L. C. Grabow, W. S. Epling and C. Paolucci, Condition-Dependent Pd Speciation and NO Adsorption in Pd/Zeolites, ACS Catal., 2020, 10, 12801–12818 CrossRef CAS.
- İ. A. Reşitoğlu, K. Altinişik and A. Keskin, The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems, Clean Technol. Environ. Policy, 2015, 17, 15–27 CrossRef.
- C. Guardiola, J. Martín, B. Pla and P. Bares, Cycle by cycle NOx model for diesel engine control, Appl. Therm. Eng., 2017, 110, 1011–1020 CrossRef CAS.
- M. Brändle and J. Sauer, Combining ab initio techniques with analytical potential functions. A study of zeolite-adsorbate interactions for NH3 on H-faujasite, J. Mol. Catal. A: Chem., 1997, 119, 19–33 CrossRef.
- C. Y. Li and L. V. C. Rees, Ion exchange, thermal stability and water desorption studies of faujasites with different Si/Al ratios, React. Polym., Ion Exch., Sorbents, 1988, 7, 89–99 CrossRef CAS.
- P. Gallezot, A. Alarcon-Diaz, J.-A. Dalmon, A. J. Renouprez and B. Imelik, Location and dispersion of platinum in PtY zeolites, J. Catal., 1975, 39, 334–349 CrossRef CAS.
- G. Maurin, D. Plant, S. Devautour-Vinot, A. Nicolas, F. Henn and J. C. Giuntini, Cation mobility upon adsorption of methanol in NaY faujasite type zeolite: A molecular dynamics study compared to dielectric relaxation experiments, Eur. Phys. J.: Spec. Top., 2007, 141, 113–116 Search PubMed.
- K. Klier, Transition-metal ions in zeolites: the perfect surface sites, Langmuir, 1988, 4, 13–25 CrossRef CAS.
| This journal is © the Owner Societies 2022 |