Protein dynamics of human serum albumin at hypothermic temperatures investigated by temperature jump†
Abstract
Human serum albumin (HSA) is the most abundant protein in human plasma. Most protein dynamics studies of HSA have been performed above the hyperthermia temperature (>42 °C), so information on the dynamics under hypothermic conditions (<35 °C) is lacking. In this work, a tryptophan-based fluorescence temperature jump system was employed to investigate the thermally-induced dynamic process of HSA at a physiological concentration of ca. 45 mg mL−1 and pH = ca. 7 upon an instantaneous temperature increase from 25 °C to 30–43 °C. The observed kinetics manifested a three-state consecutive feature, 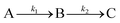 . Upon analysis with the Arrhenius model, the rate coefficients k1 and k2 manifested piecewise temperature dependence, and the turning-point temperature was found to be ca. 34 °C, coinciding with the upper bound of hypothermic temperature. Meanwhile, the corresponding activation energies of the transitions at 34–43 °C were lower than those at 30–34 °C, suggesting that protein conformational adjustments at 34–43 °C were more feasible than those at hypothermic temperatures. These observations provided a fresh viewpoint on the relationship between the energetics of protein dynamics and the apparent functioning of a given protein at the molecular level.
. Upon analysis with the Arrhenius model, the rate coefficients k1 and k2 manifested piecewise temperature dependence, and the turning-point temperature was found to be ca. 34 °C, coinciding with the upper bound of hypothermic temperature. Meanwhile, the corresponding activation energies of the transitions at 34–43 °C were lower than those at 30–34 °C, suggesting that protein conformational adjustments at 34–43 °C were more feasible than those at hypothermic temperatures. These observations provided a fresh viewpoint on the relationship between the energetics of protein dynamics and the apparent functioning of a given protein at the molecular level.



 Please wait while we load your content...
Please wait while we load your content...