Is ethaline a deep eutectic solvent?†
Abstract
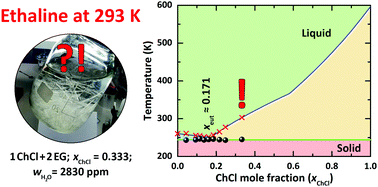
With the present contribution we clarify the phase behaviour of choline chloride (ChCl) + ethylene glycol (EG) mixtures for ChCl mole fractions (xChCl) less than 0.333 and temperatures below 323 K by providing melting points obtained by differential scanning calorimetry for samples containing <300 ppm of water. We show that ethaline, the ChCl : EG mixture of molar ratio 1 : 2 that is usually believed to be the composition of the eutectic point, actually lies in the ChCl-saturated region of the {ChCl + EG} phase diagram. The real eutectic point was found to be at the 1 : 4.85 molar ratio of ChCl : EG (xChCl = 0.171) which is characterized by a melting point of 244 K. This temperature is only 16 K below the melting point of neat EG. Thus, neither from its particular composition nor from the observed melting-point depression of {ChCl + EG} mixtures can ethaline be considered a “deep eutectic solvent”. Surprisingly, despite ChCl being an electrolyte dissolved in EG, the phase diagram is that of an ideal binary mixture.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...