Open Access Article
Open Access ArticleProbing the mechanisms of enhanced crystallisation of APS in the presence of ultrasound†
Peter R.
Birkin
 *a,
Jack J.
Youngs
*a,
Jack J.
Youngs
 a,
Tadd T.
Truscott
a,
Tadd T.
Truscott
 b and
Silvana
Martini
b and
Silvana
Martini
 c
c
aDepartment of Chemistry, University of Southampton, Southampton, UK, SO17 1BJ, UK. E-mail: prb2@soton.ac.uk
bDepartment of Mechanical and Aerospace Engineering, Utah State University, Logan, UT, 84322-4130, USA
cDepartment of Nutrition, Dietetics, and Food Sciences, Utah State University, Logan, UT, 84322-8700, USA
First published on 4th May 2022
Abstract
Understanding the origins of the enhancement of crystallisation of a lipid (all-purpose shortening, APS) through the application of ultrasound is a fundamental pre-requisite for the exploitation of this technique in a wider context. To this end, we show here a number of measurements designed to probe the mechanisms responsible for this effect. For example, we show how the type of bubble cluster, produced at the sound source, alters the bubble population and residency time. In addition, to probe the various contributions to the enhanced crystallisation rate, isolation of the cluster environment below the piston like emitter (PLE) used as the ultrasonic source was shown to reduce the enhancement observed, but did not remove it entirely. This implied that the exposure of the liquid to pressure shocks and the environment around the cluster has a positive effect on the crystallisation kinetics. In turn the addition of extra seed crystals and mechanical agitation also enhances the rate of crystallisation. Finally, the time at which ultrasonic irradiation of the fluid is applied is shown to alter the kinetics observed. These observations suggest that two components are important: large bubble populations and mechanical effects on pre-existing crystals. These findings suggest that maximising these effects could be an eloquent way to enhance and control the material characteristics of materials produced in this manner.
Introduction
The effects of cavitation on the physical and chemical properties of a system are often complex with many different factors to consider. For example, chemical processes can be affected through the sonochemical hotspot,1–3 surface activation or even mechanochemical effects.4 Physical processes can be equally dramatic with the generation of intense shockwaves, the erosion of surfaces,5 shear driven processes, bubble oscillation6,7 with associated forced convection (sometimes referred to as microstreaming7,8) and the emission of short duration light flashes9,10 noted. These complexities have been investigated with a multidisciplinary approach. However, many of these investigations have centred on aqueous environments. While many non-aqueous environment studies are noteworthy, this area is perhaps less well understood. In this context, lipid systems have received some attention.11–13 While general lipid chemistry is of interest, food lipids are particularly interesting as they make a considerable contribution to the global food industry.14 One such area of interest has been reports of the effects of cavitation on the solidification of lipids. In this case, high-intensity ultrasound, or HIU, can be shown to have beneficial effects in terms of the acceleration of the crystallisation kinetics as well as alteration of the material characteristics of the resultant solid. The root of the effect has often been attributed to cavitation. While this may be true, considering the complex environment, from a chemical and physical perspective, much more work is required to unravel the exact cause of the changes observed. This is not only illustrative from an academic perspective, but the understanding gathered by these studies will have many potential applications. For example, rather than the ad hoc application of sound energy at a particular frequency in a wide range of cell configurations, understanding what effect is necessary will mean that the approach can be tailored so that the positive effects are maximised while the negative effects (e.g., the oxidation15 of the chemical species within the media) are minimised. To gather the information necessary, an array of experimental techniques is desirable. These techniques must have a number of characteristics including the temporal resolution, the sensitivity, and the ability to operate with an analytical basis in lipid systems exposed to HIU. Techniques such as high-speed imaging, light scattering16 (both in situ and of the resultant material), acoustic measurements, thermal profiling16 and characterisation of the resultant materials can be highly rewarding in this context. For example, these approaches have been used with food oils and indicated a rich and interesting set of conditions are present. This includes cluster dynamics, high-speed streamers, bifurcated streamers, local heating and altered crystallisation kinetics (and material changes) related to the dynamics of the bubbles present. Central to this is the cluster that forms at the eroded tip deployed in the media. This fascinating phenomenon appears to be related to the effects observed.While much progress has been made, several key questions remain. First, why does cavitation generated in this manner alter the kinetics of crystallisation so effectively? Second, what are the root causes of this alteration? Third, why does the particular cluster present give rise to varying degrees of effect observed? Some insight into the answers to these questions will now be given using experimental observations related to the lifetime of bubbles within the media and the effect of different treatment protocols on the crystallisation kinetics.
Methods
Materials
Experiments were performed using sunflower oil (SFO) in the isolation cell and all-purpose shortening (APS, Bunge). Acrylic (ACR) cells were obtained from Striking Displays and ABS from Verbatim.Crystallisation experiments
APS samples were melted in a microwave oven and then stored in an oven at 80 °C for 30 minutes to remove residual crystal memory. The melted sample (100 g) was transferred to a double-walled thermostatic crystallisation cell connected to an external water bath (Grant, LT ecocool 100), set to the relevant crystallisation temperature (Tc). The cooling regime is shown in Fig. S1 (ESI†) for reference. To measure the crystallisation kinetics, samples were transferred to p-NMR tubes, previously pre-heated to the crystallisation temperature. The solid fat content (SFC) of the samples was then monitored by recording the SFC vs. time using NMR Minispec mq20 series (Bruker, California, USA). The data was fitted to the Gompertz model17 (see ref. 18 for further details).Optical sensor construction calibration and operation
The construction and calibration of the optical transmission sensor (OTS) has been described previously.19 In brief this sensor was fabricated using a glass capillary (1.6 mm internal diameter). A photodiode and LED were used as the detector and emitter respectively. These were placed on opposite sides of the glass capillary set in Epofix resin (Struers) and polished down to reveal a channel. A simple amplifier16 (gain 106) was used to detect the signal from the photodiode which was recorded on a DAQ system (USB204, Measurement computing). To draw fluid containing the generated bubbles through the OTS, a pressure rig was deployed were a gas syringe (120 ml, Rapid Electronics) and mass was used to generate a constant reduced pressure compared to the atmospheric pressure.20 The exact pressure difference was monitored using an EBRO vacuum meter (VM 2000) and is reported in each figure legend as appropriate. The data was captured using software written in VB2010 incorporating Measurement studio (National Instruments). Data analysis (e.g., for peak finding) was undertaken using Matlab.HIU setup
Cavitation was generated using a ‘piston like emitter’ (PLE, Misonix, S3000, f = 20 kHz) immersed in the relevant media here an oil (SFO or APS). A 3.2 mm diameter eroded16 tip was employed in all cases. The tip of the PLE was not polished as this was found not to be conducive to the formation of stable clusters in these oil systems (see ref. 16). The cluster characteristics have been detailed before and are generated at a set power level.18 These clusters are denoted by f/N where f is the PLE frequency and N an integer value.16 The OTS was positioned with respect to the PLE tip using micrometers and stages (Thor labs).Crystal microstructure
A polarised light microscope (PLM-Olympus BX 41, Tokyo, Japan) fitted with a digital camera (Infinity 2, Lumenera Scientific, Ottawa, Canada) was used to record the crystal microstructure of crystallised lipid material. Images were recorded approximately 20 minutes after initial sample cooling.Viscoelastic properties
A magnetic bearing rheometer (model AR-G2, TA Instruments, New Castle, DE, USA) was used to measure the elasticity of crystallised samples after approximately 60 minutes of crystallisation. A parallel plate geometry (40 mm diameter) was employed and maintained at the relevant Tc (26 °C or 30 °C). Depending upon the relative hardness of the sample, a gap of between 500–1000 μm was used. Oscillatory tests were performed by a strain sweep step from 0.0008–10% of strain and equilibration time of 1 minute. Viscoelastic parameters such as the storage modulus (G′) were recorded at 0.01% of strain.Hardness
The hardness of the crystallised material was evaluated by texture profile analysis (TPA) using a Texture Analyser (model TA, XT Plus, Texture Technologies Corp., Scarsdale, NY, USA). Samples were transferred to labelled plastic tubes (1 cm diameter) after 60 minutes and stored at 5 °C for 48 h. Chilled samples were cut to a height of approximately 1 cm, placed on the sample platform and a two-step compression with a 5 cm diameter cylindrical probe performed. A constant speed of 5 mm s−1 and compression strain of 25% were employed. Note, data was not recorded at 60 minutes after processing as the lipid sample was found to be too soft to measure its hardness at this time.Results and discussion
One of the fundamental unknowns related to the effects of ultrasound on any system, is the population of bubbles present within the media. This is key as the interaction of these bubbles with the sound field drives many of the physical and chemical processes that occur. To characterise this population and see how this changed over time and with the type of cavitation environment present, an optical transmission sensor was deployed (see ref. 19 for further details). Here, the response of a simple photodiode was monitored as a function of time and used to estimate the size and number of the bubbles present in the media as the oil is drawn through a capillary.In these experiments the population of bubbles within the media was followed as a function of time over a ∼300 s time window after the exposure of the liquid to HIU was terminated. This is limited by the crystallisation of the media. Fig. 1 shows the results of these experiments for an APS sample exposed to different cavitation environments (specifically different cluster types generated by applying different HIU power levels to the sample). Fig. 1(a), for example, shows the response for the bifurcated streamer (BiS) generated under these conditions. In this case the smallest size range was observed. Note, the sensor and analysis used limits the lower detectable radius to the order of 45 μm. Hence, bubbles smaller that this will not be detected. This is particularly relevant to the BiS event as this appears to produce a mist of small bubbles. Fig. 1 also shows that as the power of the HIU source was increased the number and size of the detectable bubbles within the fluid increased. Fig. 2 shows how the number of events (determined as detectable transients above the baseline) within the media changed as the cluster type and the temperature of the APS sample were altered. It is also important to note that these measurements were performed after the HIU source had been terminated. This indicates that the bubble population is long lived and of varied size.
Turning to the lifetime of the events within the media, Fig. 2 shows how this varies with both the temperature and the HIU power (and type of cluster dynamics generated). Fig. 2 shows that the number of events rises with HIU power and the bubble retention time (defined as the time when transients above the baseline could be recorded) climbs as the HIU power was increased. The temperature of the media also has an effect. For example, Fig. 2(a) shows that the numbers of bubbles recorded are higher at lower temperatures while the retention time was generally smaller at higher temperature. This observation is presumably a result of differing generation conditions and retention effects within the lipid. The decreased viscosity of the lipid at higher temperature is expected to allow bubbles, particularly large ones in the population, to be lost through buoyancy effects over the time course of the experiment. For example, in APS the terminal velocity21–24 of a bubble with a radius of 100 μm can be calculated to be ∼0.91 mm s−1 at 30 °C while it is ∼0.64 mm s−1 at 20 °C (see SI data, Fig. S3, ESI†). In turn the smaller the bubble, the slower its rise time (see SI data, Fig. S2, ESI†). This implies that small bubbles produced in a lipid are slow moving over the timeframe of these experiments. However, other effects are worth consideration. For example, dissolution of bubbles in a gas saturated media is driven by a number of effects including the Ostwald coefficient25 of the gas in question, the diffusion coefficient of the gas in the media and the surface tension26 of the gas/liquid interface. Epstein and Plesset reported that the dissolution time under these conditions can be calculated.27 If we compare a bubble with a radius of 10 μm in water and oil, the dissolution times are significantly different (see SI data, Fig. S4, ESI†). For example, in water this can be calculated as 7.59 s while in oil the same sized bubble will have a dissolution lifetime of 168 s. This longer lifetime (which may be extended through shell effects28 in the crystallising media) of these particularly small bubbles suggests that cluster environments that produce these (e.g., the BiS system) may be more efficient in terms of primary nucleation processes in these lipid environments.
If the bubble population alone is the important factor, the results contained in Fig. 2 suggest that the f/4 cluster should behave very similarly to the BiS system. However, for the BiS event (produced at the lowest power, 10 W) an unusually high crystallisation rate was observed. This suggests that there may be other effects that come into play. In order to probe these effects, a series of experiments were performed. In the first set, the cluster was isolated from the crystallising media to separate the cluster and its bubble population from the main crystallising media. In the second set of experiments, the time of HIU application and the effect of added crystals were investigated.
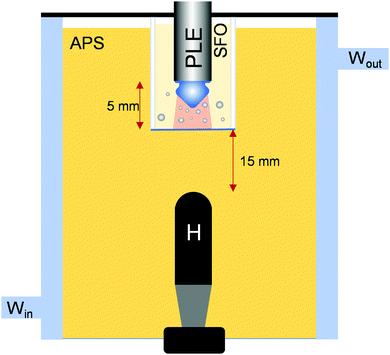
To isolate the cluster/HIU tip from the crystallising media a dual compartment cell was utilised. Fig. 3 shows a schematic representation of the cell employed. This cell had two compartments. The PLE, cluster and associated bubbles were contained in the upper compartment. This contained sunflower oil. The lower, main chamber contained the APS sample. Fig. 4 shows the response of the hydrophone as the conditions were altered. In the absence of the upper containment chamber, Fig. 4 shows a typical response for the effect of HIU on a crystallising APS sample. The amplitude of the signal ( ) falls with time. Fig. 4 also shows the effect of the upper chamber and the materials that are deployed. If the walls of the upper chamber are all 3 mm acrylic, the signal detected by the hydrophone (
) falls with time. Fig. 4 also shows the effect of the upper chamber and the materials that are deployed. If the walls of the upper chamber are all 3 mm acrylic, the signal detected by the hydrophone ( ) is severely reduced in comparison to the chamber free example. This indicates that as well as containing the bubble population and cluster in the upper chamber, sound propagation into the APS sample is severely restricted. However, if the base of the containment cell is changed to 0.3 mm ABS, the signal recorded by the hydrophone (
) is severely reduced in comparison to the chamber free example. This indicates that as well as containing the bubble population and cluster in the upper chamber, sound propagation into the APS sample is severely restricted. However, if the base of the containment cell is changed to 0.3 mm ABS, the signal recorded by the hydrophone ( ) is significantly higher. This change in behaviour is likely to be as a result of the material characteristics including the acoustic impedance of the materials involved and their relative attenuation effects. For example, the acoustic impedance of ACR is higher than ABS which would suggest more sound energy was reflected from the lipid/ACR interface. In addition, the attenuation coefficient of ABS is ∼11 dB cm−1vs. ∼12.4 dB cm−1 for ACR.29 These properties, in addition to the change of thickness of the samples, will contribute significantly to the observed change in sound transmission into the lower chamber. The effect on the crystallisation kinetics of the APS sample was also assessed under these conditions. Table 1 shows how the conditions employed affected the process.
) is significantly higher. This change in behaviour is likely to be as a result of the material characteristics including the acoustic impedance of the materials involved and their relative attenuation effects. For example, the acoustic impedance of ACR is higher than ABS which would suggest more sound energy was reflected from the lipid/ACR interface. In addition, the attenuation coefficient of ABS is ∼11 dB cm−1vs. ∼12.4 dB cm−1 for ACR.29 These properties, in addition to the change of thickness of the samples, will contribute significantly to the observed change in sound transmission into the lower chamber. The effect on the crystallisation kinetics of the APS sample was also assessed under these conditions. Table 1 shows how the conditions employed affected the process.
| Conditions | a G (%) | μ max (% min−1) | λ (min) |
|---|---|---|---|
| wHIU – no inner cell | 7.7 ± 0.3a,b | 0.63 ± 0.02c | 14.2 ± 0.6b |
| wHIU – ACR inner cell | 8.0 ± 0.4a,b | 0.30 ± 0.03a | 27.1 ± 0.9a |
| wHIU – ABS inner cell | 8.1 ± 0.1a | 0.43 ± 0.05b | 27.0 ± 0.5a |
| woHIU – ABS inner cell | 8.2 ± 0.2a | 0.27 ± 0.01a | 28.4 ± 0.7a |
| woHIU – no inner cell | 7.3 ± 0.5b | 0.29 ± 0.04a | 29 ± 1a |
These results were obtained from analysing the solid fat content as a function of time over a 60 minutes period using a pNMR approach. The results in Table 1 show that, while we restrict the cluster and bubble population to the upper chamber, transmission of sound to the APS has an effect on the crystallisation kinetics with the degree of transmission affecting the enhancement observed. First, the ABS cell gave a greater enhancement compared to the acrylic case and the without HIU (woHIU) controls in agreement with the sonic transmission data shown in Fig. 4. Second, and significantly, the isolation of the bubble population from the main APS chamber did not completely remove the enhanced crystallisation kinetics observed. This suggests that the progress of the shock like emission into the sample had an effect. This is presumably linked to the action of this physical effect on the crystallites within the media. Third, this physical effect is supported by observing the changes in the crystal structure of the resultant material. Fig. 5 shows the microstructure of samples taken under the various conditions explored.
Fig. 5 shows that the microstructure of the sample changes from the control woHIU towards the wHIU (no isolation cell) as expected from the kinetics data reported in Table 1. These results suggest that a clear effect of ultrasound is associated with the progression of the sound waves through the media. It is postulated here that the intense ‘shock like’ emissions which are associated with the cluster collapse at the PLE tip cause fragmentation of the pre-existing crystals in the sample and lead to secondary nucleation effects. To support this, a set of experiments were performed to investigate the effect of the presence of pre-existing nuclei on the process. Fig. 6 shows the acoustic data gathered from samples cooled to a set temperature and then exposed to ultrasound. However, the delay between the sample reaching the desired crystallisation temperature (here 30 °C) and the application of HIU was varied.
Fig. 6 shows that as the time period resting at 30 °C was increased (see Fig. S1, ESI† for cooling profile), the attenuation rate of the acoustic signal detected by the hydrophone increased.
The effective attenuation coefficient of the crystallising media (see ref. 18 for details) was calculated by fitting the hydrophone positive envelope to a second order model. Table 2 shows how the attenuation coefficient changes in response to the time at which HIU was applied to the sample. The data suggests that the attenuation of the hydrophone signal depends strongly on the time period spent at the crystallisation temperature. In turn, this indicates that the attenuation of the signal is largely in response to the interaction of the HIU with the crystal population. As a result, little attenuation was observed for the data recorded at 7 minutes. This is unsurprising as the temperature of the sample was ∼35 °C at this point. Under these conditions no crystals are expected and the attenuation coefficient, which appears strongly linked to the crystal population, is close to zero. However, as the sample temperature was reduced further, a greater crystal population (which has been associated with an increased effectiveness of HIU30–32) and hence acoustic attenuation was observed.
| t cooling to 30 °C min−1 | k 2/V−1 s−1 | r 2 |
|---|---|---|
| 7 | 0.03 ± 0.03a | 0.95 |
| 10 | 0.13 ± 0.05b | 0.97 |
| 15 | 0.28 ± 0.02c | 0.97 |
| 18 | 0.31 ± 0.02c | 0.97 |
Turning to the kinetics of crystallisation, Table 3 shows how the different conditions affect the rates observed. These results indicate that all periods tested showed faster crystallisation kinetics than the control. Critically the measurements at 7 minutes where the sample was ∼35 °C, shows a significant improvement in the rate of crystallisation (μmax) and a reduction in the induction time (λ). This shows that in the absence of observable crystals, HIU has a positive effect on the kinetics.30–32 This presumably indicates that the bubble population is able to contribute to primary nucleation of the sample. This is supported by the lifetime measurements shown in Fig. 2 which showed that detectable bubbles last for at least 300 s (and smaller bubbles will be longer lasting in comparison). This agrees with the buoyancy and dissolution effects previously discussed. Hence the bubble population generated at 7 mins will last into the time period when crystallization occurs. This supports the primary nucleation effect within the sample. However, as the time period before the application of HIU was increased, significant attenuation of the hydrophone signal was observed (see Table 2) and a greater acceleration in the crystallisation kinetics was seen (see Table 3). This observation suggests that HIU has a significant secondary nucleation effect whereby crystals are fragmented by the conditions present (either shear effects around oscillating bubbles present or the large pressure shocks generated by the cluster collapses, supported by the results shown in Table 1).
| Conditions [temp/°C] | a G (%) | μ max (% min−1) | λ (min) |
|---|---|---|---|
| woHIU | 7.3 ± 0.5a | 0.29 ± 0.04a | 29 ± 1a |
| wHIU 7 min [35] | 7.6 ± 0.1b | 0.49 ± 0.03b | 21.5 ± 0.3b |
| wHIU 10 min [30] | 7.7 ± 0.1b | 0.52 ± 0.06b | 18.8 ± 0.3c |
| woHIU 15 min [30] | 7.7 ± 0.3b | 0.63 ± 0.02c | 14.2 ± 0.6d |
| woHIU 18 min [30] | 7.6 ± 0.4b | 0.76 ± 0.08d | 14.5 ± 0.9d |
In order to probe these conditions further, polarised light imaging of samples after different sets of conditions at the point of HIU application and after 60 minutes was undertaken. Fig. 7 shows the images obtained. These images show that no observable crystals are present at 7 min (Tm) in agreement with the acoustic attenuation data shown in Fig. 6. Fig. 7 also shows the changes in the microstructure of the sample after 60 mins was similar to the woHIU control. However, the enhanced kinetics observed at this point suggest that the primary (bubble induced) crystallisation rate does not have a marked effect on the resultant microstructure. This observation suggests that primary nucleation occurs in both the treated and untreated samples in a similar way as expected. However, as the crystal population increased (see Fig. 7, panel ‘A’, TSC, FC and XC), crystals are observed prior to the application of ultrasound. The size and number of crystals is also seen to increase as the cooling period was increased. The application of ultrasound (see Fig. 7 panel ‘B’ for the structure after 60 minutes) shows that these crystals are broken up significantly with the average crystal size reducing visibly while the packing density seems to be greater for those samples held for longer at 30 °C before the application of ultrasound (10 s, 75 W in all cases) to the system. Hence, the more delayed the application of ultrasound (and the generation of a significant crystal population) yields significant secondary30 nucleation effects further enhancing the crystallisation kinetics (see Table 3) and altering the microstructure (see Fig. 7).
It could be envisaged that this change in microstructure in response to HIU (and enhanced secondary nucleation combined with primary nucleation effects) can alter the material properties of the resultant material. Fig. 8 shows how the material characteristics are affected by these effects. First, the elastic modulus is the same for the control (wo) and 7 minute experiment. However, where secondary effects are expected (when there is a significant crystal population when the sample was exposed to ultrasound), the elastic modulus increases significantly and continues to increase as the pre-HIU crystal population increases. Second, there is an increase in the hardness of all samples when exposed to HIU which increases further as the cooling time was extended. This presumably indicates that any increase in the kinetics (both primary and secondary) affects this parameter.
The evidence presented suggests that both primary and secondary effects are triggered by the application of HIU to the APS samples with the exact combination dictated by the crystal population within the sample at the point of HIU application. To test this assertion further, a set of experiments were performed where APS crystals were added to the media (by the addition of a small quantity of APS allowed to crystallise in the absence of HIU for 25 minutes) and the effect of this addition was investigated. Table 4 shows the effect of these additions. The addition of crystals with stirring or HIU display a greater rate than HIU alone. This suggests that the crystal population and the mechanical agitation of this media plays a significant role in the enhancement of the crystallisation kinetics.33,34 The material characteristics of the samples also showed some variation. However, the HIU treated samples showed the greatest change in agreement with the similarity of the PLM images of these samples (see SI Fig. S5 and S6, ESI† respectively).
| Conditions | a G (%) | μ max (% min−1) | λ (min) |
|---|---|---|---|
| woHIU | 7.3 ± 0.5a | 0.29 ± 0.04a | 29 ± 1a |
| 10 cm3 crystal addition, 200 rpm | 7.8 ± 0.1b | 0.77 ± 0.03b | 18.4 ± 0.3b |
| 10 cm3 crystal addition, wHIU | 7.6 ± 0.2b | 0.72 ± 0.05b | 14.3 ± 0.4c |
| wHIU | 7.7 ± 0.4b | 0.63 ± 0.08c | 14.2 ± 0.6c |
Turning to the effect of stirring the sample (and the induced shear, which has been shown to be effective at accelerating crystallisation of IESBO34), a set of experiments were performed to probe this effect. Table 5 shows the results of this investigation. These results indicated that mild stirring (200 rpm) was not effective in enhancing the crystallisation process while higher rates (600 rpm) showed a significant increase.
| Conditions | a G (%) | μ max (% min−1) | λ (min) |
|---|---|---|---|
| woHIU | 7.3 ± 0.5a | 0.29 ± 0.04a | 29 ± 1a |
| 200 rpm | 8.1 ± 0.2b | 0.33 ± 0.04a | 29 ± 2a |
| 600 rpm | 7.9 ± 0.3b | 0.42 ± 0.06b | 23 ± 2b |
| wHIU | 7.7 ± 0.3b | 0.63 ± 0.02c | 14.2 ± 0.6c |
This suggests that mechanical agitation, if of sufficient intensity, is effective at accelerating the crystallisation process. Interestingly, the velocity of the stirrer tips for 600 rpm can be estimated at ∼0.6 m s−1. This is slightly lower than the streamer velocity produced by the cluster.16 However, the rates of crystallisation are far lower than for the HIU case suggesting that it is not only the flow but the other physical effects of the cluster (shocks, local shear etc.) which need to be considered.
The results presented here all suggest that the effect of ultrasound on fat crystallisation is multifaceted. The cavitation environment can generate primary nucleation sites through the production of a significant long lived bubble population. In turn the action of the cluster, which produces a strong and regular shock like emission, further accelerate the crystallisation kinetics through, presumably, secondary nucleation effects. At this point some regard to the structure and nature of the APS sample should be noted.
It has been shown that crystal fragmentation35–37 can be generated in a variety of systems and this process is related to the mechanical properties of the sample. For example, Kim et al. showed that salt crystals could be fragmented in an ultrasonic field with the rate of fragmentation related to the Vickers hardness and Youngs modulus of the material employed.36 If we consider APS in comparison, it is interesting to note that the crystals38,39 (or spherulites) are really conglomerates of smaller TAG nanocrystals held together by weak van der Waals forces.39 Correspondingly, the Youngs modulus38 of a single spherulite is nearly 105 smaller38 at ∼1 MPa compared to a material like NaF (77.5 GPa), for example. Hence, we can expect that such APS spherulites will be fragmented easily in comparison by the forces generated by the HIU treatment of the sample. This agrees with the observation in the data provided above. However, further insight is possible. The bifurcated streamer (BiS) system which can be generated in these oil systems was shown18 to be highly active in the acceleration of APS crystallisation, in excess of what would be expected on a power basis alone (compared to other clusters). The reasons for this are now, perhaps, more apparent.
The BiS system possess two of the key components that have been shown to enhance APS crystallisation. First, it generates a set of small gas bubbles which populate the fluid. Thus, this dual streamer could be expected to enhance primary nucleation events in excess of a single cluster stream. Second, the strong pulse like shock generated by the cluster collapse (the strongest globally measurable emission in the system) is more frequent in comparison to all other clusters. Indeed, the BiS's anatomy consists of two clusters oscillating at an f/2 period but 180° out of phase with one another. This essentially results in a shock emission into the sample at f, the ultrasonic source, (and hence a weak emission). The fluid, bubble population and crystals are hence exposed to a pressure shock every cycle of the source which is more often than any other cluster environment measured for these oil clusters. The shear forces around the clusters themselves may also play a role, as yet to be quantified. However, the BiS event has two clusters so may also be at an advantage here. Finally, the bubbles within the media will also be an active part of the system with their motion, presumably classed as non-inertial cavitation, in the associated sound field will produce local shear40 through their motion and the associated microstreaming.7 Hence, these events will contribute to the secondary nucleation effects. Fig. 9 brings the observed components together and highlights the proposed important factors and the characteristic of each environment.
Finally, the contribution of the relative shear of the differing components, whether that is the fluid driven in the streamer, the motions around the cluster itself or the motion of gas bubbles in response to the streamer or the shock emission into the bulk, all need to be assessed and quantified. This remains a fascinating question in relation to the relative effects of the physical processes within the crystallising media.
Conclusions
A number of factors have been explored. The evidence suggests that both primary and secondary nucleation is important in assessing the acceleration of crystallisation of APS as a result of HIU action. However, this effect is clearly multifaceted with contributions from more than one origin likely. To this end the abnormally high rate of crystallisation in the presence of the BiS event can now be further understood through its unusual characteristics. Specifically, this system produces many small bubbles (and hence can be an effective primary nucleation source) and exposes the fluid to more frequent pressure shocks which accelerate secondary nucleation effects.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by Agriculture and Food Research Initiative (AFRI) Grant No. 2017-67017-26476 from the USDA National Institute of Food and Agriculture, Improving Food Quality-A1361. This paper was approved by the Utah Agricultural Experiment Station as Paper Number 9545.References
- K. S. Suslick, D. A. Hammerton and R. E. Cline, The sonochemical hotspot, J. Am. Chem. Soc., 1986, 108, 5641–5642 CrossRef CAS.
- K. S. Suslick, Sonochemistry, Science, 1990, 247, 1439–1445 CrossRef CAS.
- K. S. Suslick, S. J. Doktycz and E. B. Flint, On the origin of sonoluminescence and sonochemistry, Ultrasonics, 1990, 28, 280 CrossRef CAS.
- J. N. Brantley, K. M. Wiggins and C. W. Bielawski, Polymer mechanochemistry: The design and study of mechanophores, Polym. Int., 2013, 62, 2–12 CrossRef CAS.
- P. R. Birkin, R. O’Connor, C. Rapple and S. S. Martinez, Electrochemical measurement of erosion from individual cavitation events generated from continuous ultrasound, J. Chem. Soc., Faraday Trans., 1998, 94, 3365–3371 RSC.
- Y. E. Watson, P. R. Birkin and T. G. Leighton, Electrochemical detection of bubble oscillation, Ultrason. Sonochem., 2003, 10, 65–69 CrossRef CAS PubMed.
- S. A. Elder, Cavitation microstreaming, J. Acoust. Soc. Am., 1958, 31, 54 CrossRef.
- F. R. Young, Cavitation, Imperial College Press, London, 1999 Search PubMed.
- L. A. Crum and G. T. Reynolds, Sonoluminescence produced by ‘stable’ cavitation, J. Acoust. Soc. Am., 1985, 78, 137–139 CrossRef CAS.
- A. J. Walton and G. T. Reynolds, Sonoluminescence, Adv. Phys., 1984, 33, 595–660 CrossRef CAS.
- S. Martini, R. Tejeda-Pichardo, Y. Ye, S. G. Padilla, F. K. Shen and T. Doyle, Bubble and crystal formation in lipid systems during high-intensity insonation, J. Am. Oil Chem. Soc., 2012, 89, 1921–1928 CrossRef CAS.
- S. Martini, in Sonocrystallization of Fats, Springer International Publishing, New York, 2013, pp. 41–62 Search PubMed.
- A. H. Suzuki, J. Lee, S. G. Padilla, S. Martini, A. H. Suzuki, J. Lee and S. G. Padilla, Altering functional properties of fats using power ultrasound, J. Food Sci., 2010, 75, E208–E214 CrossRef CAS.
- Acumen Research and Consulting (accessed 22/09/2021).
- M. E. Abdelsalam and P. R. Birkin, A study investigating the sonoelectrochemical degradation of an organic compound employing Fenton's reagent, Phys. Chem. Chem. Phys., 2002, 4, 5340–5345 RSC.
- P. R. Birkin, T. M. Foley, T. T. Truscott, A. Merritt and S. Martini, Cavitation clusters in lipid systems – surface effects, local heating, outgassing and streamer formation, Phys. Chem. Chem. Phys., 2017, 19, 6785–6791 RSC.
- I. Foubert, K. Dewettinck and P. A. Vanrolleghem, Modelling of the crystallization kinetics of fats, Trends Food Sci. Technol., 2003, 14, 79–92 CrossRef CAS.
- J. J. Youngs, P. R. Birkin, J. Lee, T. T. Truscott and S. Martini, Enhanced crystallisation kinetics of edible lipids through the action of a bifurcated streamer, Analyst, 2021, 146, 4883–4894 RSC.
- P. R. Birkin, J. J. Youngs, T. T. Truscott and S. Martini, Development of an optical flow through detector for bubbles, crystals and particles in oils, Phys. Chem. Chem. Phys., 2022, 24, 1544–1552 RSC.
- P. R. Birkin, S. Linfield, G. Denuault, R. Jones, J. J. Youngs and E. Wain, An analytical differential resistance pulse system relying on a time shift signal analysis-applications in coulter counting, ACS Sens., 2019, 4, 2190–2195 CrossRef CAS PubMed.
- R. Clift, J. R. Grace and M. E. Weber, Bubbles, Drops, and Particles, Dover Publications Inc., New York, 2005 Search PubMed.
- N. P. Brandon and G. H. Kelsall, Growth kinetics of bubbles electrogenerated at microelectrodes, J. Appl. Electrochem., 1985, 15, 475–484 CrossRef CAS.
- N. P. Brandon, The growth kinetics and interfacial properties of electrogenerated bubbles, PhD thesis, Imperial College, 1985 Search PubMed.
- G. H. Kelsall, S. Tang, A. L. Smith and S. Yurdakul, Measurement of rise and electrophoretic velocities of gas bubbles, J. Chem. Soc., Faraday Trans., 1996, 92, 3879 RSC.
- R. Battino, T. R. Rettich and T. Tominaga, The solubility of nitrogen and air in liquids, J. Phys. Chem. Ref. Data, 1984, 13, 563 CrossRef CAS.
- W. Duangsuwan and U. Tuzun, The dynamics of single air bubbles and alcohol drops in sunflower oil at various temperatures, AIChE J., 2011, 57, 897–910 CrossRef CAS.
- P. S. Epstein and M. S. Plesset, On the Stability of Gas Bubbles in Liquid-Gas Solutions, J. Phys. Chem., 1950, 18, 1505–1509 CrossRef CAS.
- M. A. Borden and M. L. Longo, Dissolution behavior of lipid monolayer-coated, air-filled microbubbles: Effect of lipid hydrophobic chain length, Langmuir, 2002, 18, 9225–9233 CrossRef CAS.
- Onda, Acoustic Properties of Plastics, 2003.
- Y. Ye, A. Wagh and S. Martini, Using high intensity ultrasound as a tool to change the functional properties of interesterified soybean oil, J. Agric. Food Chem., 2011, 59, 10712 CrossRef CAS PubMed.
- A. H. Suzuki, J. Lee, S. G. Padilla and S. Martini, Altering functional properties of fats using power ultrasound, J. Food Sci., 2010, 75, E208–14 CrossRef CAS PubMed.
- S. Martini, A. H. Suzuki and R. W. Hartel, Effect of high intensity ultrasound on crystallization behavior of anhydrous milk fat, J. Am. Oil Chem. Soc., 2008, 85, 621–628 CrossRef CAS.
- J. V. Kadamne and S. Martini, Sonocrystallization of interesterified soybean oil with and without agitation, J. Am. Oil Chem. Soc., 2018, 95, 571–582 CrossRef CAS.
- R. C. Silva, J. Lee, V. Gibon and S. Martini, Effects of high intensity ultrasound frequency and high-speed agitation on fat crystallization, J. Am. Oil Chem. Soc., 2017, 94, 1063–1076 CrossRef CAS.
- B. W. Zeiger and K. Suslick, Sonofragmentation of molecular crystals: Observations and modeling, Proc. Meet. Acoust., 2013, 19, 14530–14533 Search PubMed.
- H. N. Kim and K. S. Suslick, Sonofragmentation of ionic crystals, Chem. – Eur. J., 2017, 23, 2778–2782 CrossRef CAS PubMed.
- J. R. G. Sander, B. W. Zeiger and K. S. Suslick, Sonocrystallization and sonofragmentation, Ultrason. Sonochem., 2014, 21, 1908–1915 CrossRef CAS PubMed.
- H. Y. Yoshikawa, D. A. Pink, N. C. Acevedo, F. Peyronel, A. G. Marangoni and M. Tanaka, Mechanical response of single triacylglycerol spherulites by using microcolloidal probes, Chem. Lett., 2017, 46, 599–601 CrossRef CAS.
- N. C. Acevedo, F. Peyronel and A. G. Marangoni, Nanoscale structure intercrystalline interactions in fat crystal networks, Curr. Opin. Colloid Interface Sci., 2011, 16, 374–383 CrossRef CAS.
- J. A. Rooney, Hemolysis near an ultrasonically pulsating bubble, Science, 1970, 169, 869–871 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d1cp05701d |
| This journal is © the Owner Societies 2022 |