Open Access Article
Open Access ArticleMultipole-moment effects in ion–molecule reactions at low temperatures: part II – charge–quadrupole-interaction-induced suppression of the He+ + N2 reaction at collision energies below kB·10 K
Valentina
Zhelyazkova
 ,
Fernanda B. V.
Martins
,
Fernanda B. V.
Martins
 ,
Matija
Žeško
and
Frédéric
Merkt
,
Matija
Žeško
and
Frédéric
Merkt
 *
*
Laboratory for Physical Chemistry, ETH Zurich, CH-8093 Zurich, Switzerland. E-mail: merkt@phys.chem.ethz.ch
First published on 20th January 2022
Abstract
We report on an experimental and theoretical investigation of the He+ + N2 reaction at collision energies in the range between 0 and kB·10 K. The reaction is studied within the orbit of a highly excited Rydberg electron after merging a beam of He Rydberg atoms (He(n), n is the principal quantum number), with a supersonic beam of ground-state N2 molecules using a surface-electrode Rydberg–Stark decelerator and deflector. The collision energy Ecoll is varied by changing the velocity of the He(n) atoms for a fixed velocity of the N2 beam and the relative yields of the ionic reaction products N+ and N2+ are monitored in a time-of-flight mass spectrometer. We observe a reduction of the total reaction-product yield of ∼30% as Ecoll is reduced from ≈kB·10 K to zero. An adiabatic capture model is used to calculate the rotational-state-dependent interaction potentials experienced by the N2 molecules in the electric field of the He+ ion and the corresponding collision-energy-dependent capture rate coefficients. The total collision-energy-dependent capture rate coefficient is then determined by summing over the contributions of the N2 rotational states populated at the 7.0 K rotational temperature of the supersonic beam. The measured and calculated rate coefficients are in good agreement, which enables us to attribute the observed reduction of the reaction rate at low collision energies to the negative quadrupole moment, Qzz, of the N2 molecules. The effect of the sign of the quadrupole moment is illustrated by calculations of the rotational-state-dependent capture rate coefficients for ion–molecule reactions involving N2 (negative Qzz value) and H2 (positive Qzz value) for |J, M〉 rotational states with J ≤ 5 (M is the quantum number associated with the projection of the rotational angular momentum vector ![[J with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004a_20d1.gif) on the collision axis). With decreasing value of |M|,
on the collision axis). With decreasing value of |M|, ![[J with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004a_20d1.gif) gradually aligns perpendicularly to the collision axis, leading to increasingly repulsive (attractive) interaction potentials for diatomic molecules with positive (negative) Qzz values and to reaction rate coefficients that decrease (increase) with decreasing collision energies.
gradually aligns perpendicularly to the collision axis, leading to increasingly repulsive (attractive) interaction potentials for diatomic molecules with positive (negative) Qzz values and to reaction rate coefficients that decrease (increase) with decreasing collision energies.
1 Introduction
Experimental studies of ion–molecule reactions at low temperatures and low-collision energies are difficult because ions are easily heated up by stray electric fields and space-charge effects. Experiments combining cold ions in ion-traps and Coulomb crystals with cold molecules in slow beams are promising approaches to reach very low temperatures and collision energies in studies of ion–molecule reactions1–3 but have not yet been broadly applied. Measurements of reaction rates down to ≈10 K are possible in ion guides and traps4–7 and in uniform supersonic flows8–10 but hardly any measurements have been performed below 10 K. It is, however, below 10 K that the effects of the electrostatic interactions between the charge of the ion and the multipole moments of the molecule become dominant and are expected to strongly affect the reaction rates.11–16This article is the second in a series of three articles exploring the effects of molecular electric multipole moments on the rates of ion–molecule reactions at low energies. The first and third articles in this series present studies of the reactions of He+ with ammonia (NH3 and ND3)17 (referred to as Article I below) and methane (CH4 and CD4)18 and focus on the role of the interaction between the charge of the ion and the electric dipole and octupole moments of the neutral molecule, respectively. In the present article, the effect of the charge–quadrupole interaction is investigated with the example of the He+ + N2 reaction, for which we have measured the collision-energy dependence of the reaction rate coefficient in the range of collision energies between 0 and kB·10 K at a resolution of about kB·250 mK near Ecoll = 0.
These reactions are all fast and strongly exothermic.19,20 Consequently, their rates can be described by capture theories.11,12,21–25 At high temperatures (beyond 300 K) and collision energies, the capture rates can be approximated by the Langevin rate constant (in SI units)
 | (1) |
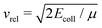 is the asymptotic relative velocity, α′ is the polarisability volume of the neutral molecule, Ze is the charge of the ion, μ is the reduced mass of the reactants and ε0 is the permittivity of vacuum.
is the asymptotic relative velocity, α′ is the polarisability volume of the neutral molecule, Ze is the charge of the ion, μ is the reduced mass of the reactants and ε0 is the permittivity of vacuum.
The effect of the electric dipole moment of the neutral molecule on ion–molecule reactions is to increase the capture rates compared to the Langevin rates at low collision energies or temperatures.8,10,17,26 The rate enhancements predicted theoretically depend strongly on the degree of sophistication of the treatment of the orientation of the molecular permanent dipole moment during the collision.22 Calculations assuming full orientation are the simplest but greatly overestimate the rate enhancements at low temperatures or collision energies. The explicit consideration of the rotational-state-dependent orientation of the molecules in the field of the ion via the Stark effect in the realm of adiabatic capture models11–14,26–29 typically leads to much less pronounced enhancements, except at the lowest collision energies or temperatures (typically below 1 K)17,26 and for rotational states that are subject to a linear negative Stark shift at low fields.17 It is only very recently that the first experimental studies of the rates of reactions between ions and polar molecules have been reported at collision energies below ≈kB·10 K, revealing the details of the rotational-state-specific rate enhancements at low collision energies for the reactions of He+ with CH3F,26 NH3 and ND3.17
The Stark effect does not only shift the rotational level energies of polar molecules but also affects the energy levels of nonpolar molecules via charge–induced-dipole, charge–quadrupole and charge–octupole interactions. The charge–induced-dipole interaction leads to an attractive long-range interaction potential scaling with the separation R between the ion and the polarisable molecule as R−4. The effect of this interaction is included in the Langevin model and leads to the well-known expression for the rate constant given by eqn (1). The effect of the charge–quadrupole interaction is insensitive to the orientation of the neutral molecule but depends on the alignment of its angular momentum vector along the collision axis, as demonstrated by Dashevskaya et al.15,16
In Article I, we have presented a general formalism, based on the earlier theoretical treatments of capture reactions by Clary and coworkers11,24,27 and Troe and coworkers,12,15,16,25,30,31 to calculate collision-energy- and temperature-dependent capture rate coefficients for reactions between ions and neutral molecules with permanent dipole, quadrupole, octupole, etc. moments, in terms of the multipole coefficients Qλ characterising the molecular charge distribution. In the present work, we apply this formalism to the charge–quadrupole interaction (λ = 2) between ions and diatomic molecules to interpret the reduction of the rate of the He+ + N2 reaction observed at low collision energies. The electric quadrupole moment of N2 can be described by a traceless, diagonal rank-2 tensor with elements Qzz = −2Qxx = −2Qyy, and a negative value of Qzz (= −1.394 D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Å).32 We present calculations of rotational-state-dependent rate coefficients kJM(Ecoll) for the low-lying |JM〉 rotational states of N2 with J ≤ 5 and highlight their dependence on the magnetic quantum number M, which is the projection of the total angular momentum of the molecule,
Å).32 We present calculations of rotational-state-dependent rate coefficients kJM(Ecoll) for the low-lying |JM〉 rotational states of N2 with J ≤ 5 and highlight their dependence on the magnetic quantum number M, which is the projection of the total angular momentum of the molecule,  , onto the quantisation axis defined by the electric field of the ion, i.e., the collision axis. These calculations demonstrate that the observed reduction of the product-ion yield at low collision energies originates from the negative Qzz value of N2. In order to illustrate the effect of the sign of Qzz on the rotational-state-specific kJM(Ecoll) rate coefficients, we also present calculations for ion–molecule capture reaction involving H2, which has a positive quadrupole moment
, onto the quantisation axis defined by the electric field of the ion, i.e., the collision axis. These calculations demonstrate that the observed reduction of the product-ion yield at low collision energies originates from the negative Qzz value of N2. In order to illustrate the effect of the sign of Qzz on the rotational-state-specific kJM(Ecoll) rate coefficients, we also present calculations for ion–molecule capture reaction involving H2, which has a positive quadrupole moment  = +0.638 D
= +0.638 D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Å,16 and for which an overall increase of the rate constant was predicted15,16 and observed33,34 at Ecoll ≤ kB·1 K. Our results are consistent with previous theoretical calculations of the dependence of the capture rate coefficients of the reactions between ions and homonuclear diatomic molecules on the sign of the qQzz product (q is the ionic charge).15,16,30,31
Å,16 and for which an overall increase of the rate constant was predicted15,16 and observed33,34 at Ecoll ≤ kB·1 K. Our results are consistent with previous theoretical calculations of the dependence of the capture rate coefficients of the reactions between ions and homonuclear diatomic molecules on the sign of the qQzz product (q is the ionic charge).15,16,30,31
The He+ + N2 reaction is an example of a charge-transfer reaction and is one of the most studied ion–molecule reactions.9,20,35–43 It is known to proceed by a near-resonance pathway44 involving the v = 3 and 4 vibrational levels of the C 2Σu+ state of N2+ and vibrationally excited levels of other electronic states.42 The reaction plays an important role in astrochemistry and in the atmospheric chemistry of the Earth45,46 and Titan.47 Molecular nitrogen is relatively abundant in dense interstellar clouds, with fractional abundances with respect to H2 estimated to be in the (4–6) × 10−6 range.48 In dense interstellar clouds, the He+ + N2 reaction is responsible for the liberation of N+, which is involved in the synthesis of NH3.49 The reaction has been studied experimentally in the 1960s and 1970s at room temperature and above using a broad range of experimental techniques – e.g., flowing-afterglow (FA),36–38 drift-tube (DT),50 ion-cyclotron-resonance (ICR),51–53 selected ion flow-drift tube (SIFT),41 slow flow drift tube (SFDT),54 and photoionisation mass spectrometry (PI-MS).35 The He+ + N2 reaction has two product channels,
| He+ + N2 → He + N + N+ | (2) |
| → He + N2+, | (3) |
Representative results on these reactions obtained in this early period are summarised in Table 1. The total reaction rate constant was measured to be in the (1.0–1.6) × 10−15 m3 s−1 range,20 slightly lower than the Langevin rate of 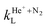 = 1.637 × 10−15 m3 s−1. Overall, these measurements did not reveal any significant dependence on the temperature or collision energy, as expected for a fast, Langevin-type reaction. In particular, no effects resulting from the charge–quadrupole interaction were detected nor discussed. The N+
= 1.637 × 10−15 m3 s−1. Overall, these measurements did not reveal any significant dependence on the temperature or collision energy, as expected for a fast, Langevin-type reaction. In particular, no effects resulting from the charge–quadrupole interaction were detected nor discussed. The N+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2+ product-ion branching ratio was determined to be 0.60[±11%]
N2+ product-ion branching ratio was determined to be 0.60[±11%]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.40[±11%], with a slight but consistent bias favouring the N+ product ion and substantial differences between individual measurements.
0.40[±11%], with a slight but consistent bias favouring the N+ product ion and substantial differences between individual measurements.
| Exp. method | N+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2+ product ratio N2+ product ratio |
k (m3 s−1) | T (K) |
|---|---|---|---|
| SIFT41 | 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
1.6(3) × 10−15 | 300 |
| FA55 | 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
1.2(4) × 10−15 | 300 |
| FA37 | 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
1.2(4) × 10−15 | 300–600 |
| DT50 | 0.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45 0.45 |
1.0(3) × 10−15 | 300–1200 |
| ICR51,52 | 0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.34 0.34 |
1.20(10) × 10−15 | 300 |
| PI-MS35 | 0.52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.48 0.48 |
1.5 × 10−15 | 300 |
| CRESU9 | 1.25 × 10−15 | 300 | |
| CRESU9 | 1.3 × 10−15 | 28 | |
| CRESU9 | 1.3 × 10−15 | 20 | |
| CRESU9 | 1.2 × 10−15 | 8 | |
| This work | 0.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 0.50 |
(0.9–1.2) × 10−15 | 0–12 |
The differences in the observed N+:N2+ product ratios are not fully understood yet. Fluorescence studies of the He+ + N2 reaction system39,43 have demonstrated that the B, C and other electronic states of N2+ are populated by charge transfer. Charge transfer to the D′ state was estimated to be 30% of that to the C state.43 Luminescence measurements in an ICR apparatus have demonstrated that charge transfer to the B state represents only ∼2% of the near-resonant charge transfer to the C state.42 Measurements of the product ratios of the He+ + 15N2 reaction at thermal energies have revealed strong isotopic effects.40,56
The complex decay dynamics of N2+ molecules excited in the energy region of the C state by charge-transfer reactions or photoexcitation has been recently discussed by Ayari et al., who have determined the radiative and predissociation lifetimes of the vibrational levels of the C state on the basis of a full set of potential energy curves obtained in high-level ab initio calculations.57 The potential energy curves for the ground state of N2 and of the ground and several electronically excited states of N2+, reproduced from ref. 57, are presented in Fig. 1. The ionisation energy of He [EI(He) = 24.58738881(3) eV or 198310.66632(20) cm−1],58 marked by a horizontal red line, lies above the first, N (4S3/2) + N+ (3P0), dissociative ionisation limit of N2 [L1 = 24.2883(10) eV or 195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 899(8) cm−1, obtained by adding the adiabatic ionisation energy of N259 and the dissociation energy of N2+];60 see short horizontal black line in the inset. Any rovibronic state of N2+ with an energy below EI(He) can be produced by charge transfer from He+ in combination with the emission of He and N2+ products of the corresponding kinetic energy. EI(He) lies 5.6 meV and 250.3 meV above the C 2Σu+(v = 4 and 3) ionisation thresholds61 of N2, respectively. These states are also located above the dissociative-ionisation threshold of N2 and can further predissociate in N (4S3/2) and N+ (3PJ) fragments.39
899(8) cm−1, obtained by adding the adiabatic ionisation energy of N259 and the dissociation energy of N2+];60 see short horizontal black line in the inset. Any rovibronic state of N2+ with an energy below EI(He) can be produced by charge transfer from He+ in combination with the emission of He and N2+ products of the corresponding kinetic energy. EI(He) lies 5.6 meV and 250.3 meV above the C 2Σu+(v = 4 and 3) ionisation thresholds61 of N2, respectively. These states are also located above the dissociative-ionisation threshold of N2 and can further predissociate in N (4S3/2) and N+ (3PJ) fragments.39
The N+ product ions formed in the He+ + N2 reaction are attributed to charge transfer to the predissociative v = 3 and 4 levels of the C state of N2+, whereas the N2+ ions result from charge transfer to longer-lived excited states of N2+. These states are either metastable and survive until the N2+ ions are detected or decay radiatively to low-lying rovibronic states of N2+ located energetically below the N+ + N dissociation threshold. The predissociation of the C 2Σu+(v = 3, 4) states most likely takes place through interactions with the 1 4Πu state.39,57,63,64 The radiative and predissociation lifetimes of the first seven vibrational states of the C 2Σu+ have been measured in ref. 65. The predissociation lifetimes of the v = 3 and 4 levels were found to be 4.2 ± 0.2 and 5.3 ± 0.3 ns, respectively. The radiative decay to the X 2Σg+(v′′) state is governed by Franck–Condon factors, which favour Δv = v − v′′ = −6 transitions.66 The radiative lifetimes were measured to be τrad,(C,v= 1,2) ≈ 78 ns,65 and determined theoretically by Ayari et al.57 to be about 60 ns for the C (v = 0–2) states. From these results, we conclude that charge transfer to the C (v = 0–2) states exclusively leads to the formation of N2+ and that the branching ratios for the formation of N2+ (ηN2+ = I(N2+)/[I(N2+) + I(N+)]) decrease to about 34% for the C (v = 3) level and to about 1% for the C (v = 4) level. The branching ratios for other states of N2+ produced by charge transfer from He+ are not known, nor is the exact nature of these states.
The bias in favour of the N+ product ions in previous FA, SIFT, and IRC measurements (Table 1), has been attributed to collision-induced dissociation of the metastable N2+ reaction products.42 This collisional dissociation results from the relatively high pressures of the background He and N2 gas in these experiments (e.g., 0.5–1 torr in the FA and SIFT experiments) and the long reaction-observation times in the ms-range. This interpretation was supported by studies of the pressure dependence of the N+/N2+ product ratio in ICR experiments,42,44,53,56 which demonstrated that the N+ product yield increases with increasing pressure and that the lowest N+/N2+ ratio (∼1.13, i.e., 0.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.47) is observed at the lowest pressure of ∼0.2 × 10−4 torr. Our experiments are carried out at low gas densities and under conditions where no secondary collisions are observed and therefore offer the possibility of establishing the low-temperature, low-pressure limit of the product branching ratios.
0.47) is observed at the lowest pressure of ∼0.2 × 10−4 torr. Our experiments are carried out at low gas densities and under conditions where no secondary collisions are observed and therefore offer the possibility of establishing the low-temperature, low-pressure limit of the product branching ratios.
| B 0 (cm−1) |
Q
zz
(D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Å) Å) |
Δα′ (a03) | α′ (a03) | μ (u) | k L (m3 s−1) | |
|---|---|---|---|---|---|---|
| He+ + N2 | 1.990 | −1.036 | 4.697 | 11.54 | 3.502 | 1.637 × 10−15 |
| D2+ + H2 | 59.3398 | 0.474 | 2.120 | 5.437 | 1.341 | 1.816 × 10−15 |
2 Experimental procedure
The experimental setup and procedure have been described in detail in Article I17 and we present here only the aspects that are specific to the study of the He+ + N2 reaction. Two short-pulse home-built valves (pulse duration ∼20 μs) are used to produce supersonic beams of He and N2. The N2 valve is heated to 330 ± 1 K, resulting in a mean forward velocity of 880 m s−1 and a rotational temperature of less than 10 K for the N2 sample. The helium valve is vertically displaced from the N2 valve and tilted at a 5° angle with respect to the vertical. A pulsed electric discharge at the He valve opening is used to populate the (1s)(2s)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3S1 state of helium (referred to as He* below). After passing through two skimmers, the He* beam is excited in the presence of an electric field to the (n, k, m) = (30, 21, 0) low-field-seeking Rydberg–Stark state. The Rydberg-helium [He(n)] beam is then deflected using a curved 50-electrode surface Rydberg–Stark decelerator26,67 and merged with the N2 beam. The He valve was cooled and temperature stabilised to 66.0 ± 0.1 K, resulting in an initial mean forward velocity of the beam of 860 m s−1. By applying the appropriate potentials to the deflector electrodes, the velocity of the Rydberg He beam can be set to any value in the range 720–1100 m s−1 and a relative-velocity distribution corresponding to a temperature of 250 mK.
3S1 state of helium (referred to as He* below). After passing through two skimmers, the He* beam is excited in the presence of an electric field to the (n, k, m) = (30, 21, 0) low-field-seeking Rydberg–Stark state. The Rydberg-helium [He(n)] beam is then deflected using a curved 50-electrode surface Rydberg–Stark decelerator26,67 and merged with the N2 beam. The He valve was cooled and temperature stabilised to 66.0 ± 0.1 K, resulting in an initial mean forward velocity of the beam of 860 m s−1. By applying the appropriate potentials to the deflector electrodes, the velocity of the Rydberg He beam can be set to any value in the range 720–1100 m s−1 and a relative-velocity distribution corresponding to a temperature of 250 mK.
The distribution of the N2 molecules in the supersonic beam over the rotational levels of the X 2Σg+(v = 0) ground state was measured by monitoring the yield of N2+ ions generated by (2 + 1) resonance-enhanced-ionisation spectroscopy of the a′′ 1Σg+ (v′ = 0, J′)–X(v = 0, J′′) transition of N2, following the procedure described in ref. 68. The distribution was found to be well described by a rotational temperature of 7.0 ± 1.0 K (see further discussion in Section 5.1 and inset of Fig. 3(b) below).
After the He(n) and the N2 beams are merged, they enter a reaction zone inside a time-of-flight mass spectrometer (TOFMS) where the reaction product ions are extracted by a pulsed electric field and accelerated towards a microchannel-plate (MCP) detector (see Fig. 1 of Article I for details). The duration of the reaction-observation window is set to be Δtr = 7 μs using a sequence of two electric-field pulses separated by Δtr. The first pulse of the sequence serves the purpose of sweeping out all ions produced before the reaction-observation window. The second pulse extracts the ions generated during the temporal observation window. The electric-field strength of the pulses is not high enough to field ionise the initially prepared He(n) atoms. Nevertheless He+ ions are detected because some of the initially populated Rydberg states undergo transitions to higher-lying Rydberg states that can be field ionised, as discussed in more detail in ref. 69.
 | ||
| Fig. 1 Potential-energy curves for the X 1Σg+ ground state of N2 from ref. 62, and for selected low-lying electronic states of N2+ (taken with permission from ref. 57). Also shown are the ionisation energy of He (EI(He)) and N2 (EI(N2)), the first dissociation limit (L1) of N2+, and the relevant vibrational levels in the C 2Σu+ state of N2+. See text for details. | ||
Typical ion time-of-flight mass spectra used to determine the N+ and N2+ product yields are presented in Fig. 2. They were obtained by averaging over 500 experimental cycles after setting the Rydberg helium velocity to vRyd = 1040 m s−1. When the He(n)-Rydberg-excitation laser is turned on (black trace), a strong He+ signal is observed at ∼1.6 μs, followed by an almost constant background signal originating from the slow ionisation of the He(n) molecules by blackbody radiation and tunnel ionisation. In addition, two peaks, corresponding to the N+ and N2+ product ions are clearly distinguishable at 2.84 μs and 3.98 μs, respectively. Small amounts of OH+ and H2O+ ions produced by Penning-ionisation reactions between He* and H2O are also detected. The kinetic energy of the N+ and N2+ molecules generated by the reactions are sufficiently low (see inset of Fig. 1) so that hardly any product ions leave the ion-extraction volume during the reaction-observation window.
When the Rydberg-excitation laser is not turned on (blue trace), the N+ and N2+ product ions are suppressed, indicating that they are produced by the He+ + N2 reaction. The He(n) ionisation background signal also disappears but the OH+ and H2O+ ion signals remain. This observation proves that these ions are produced from Penning-ionisation reactions of He* with background water in the vacuum chamber.
To determine the relative yields of the N+ and N2+ product ions, we integrate the TOF spectra over the corresponding TOF ranges, indicated by the areas shaded in pale green in Fig. 2. The integration is performed after subtraction of the background signals from Penning-ionisation processes and from the slow ionisation of He(n) Rydberg atoms by blackbody-radiation and tunnel ionisation. The collision-energy dependence of the rate coefficients is then obtained by monitoring the total product-ion yield as a function of the velocity vRyd of the He(n) beam. By varying vRyd between 720 m s−1 and 1100 m s−1 for a fixed value of the N2-beam velocity, we probe the collision-energy range between 0 and ∼kB·10 K.
The experiments are carried out under conditions where the density of N2 molecules by far exceeds that of the He(n) atoms, so that the product formation follows pseudo-first-order kinetics. Only a very small fraction (typically ≃1%) of the He(n) reacts to form product ions during the 7 μs-long reaction observation-window. Consequently, the product-ion signals directly reflect the relative rate coefficients.
3 Experimental results
3.1 Branching ratios
The relative strength of the N+ and N2+ ion signals [I(N+) and I(N2+)] is determined by integrating the ion signals in time-of-flight mass spectra such as those displayed in Fig. 2 over the corresponding time-of-flight ranges. The ratio of product-ion signal I(N+)/I(N2+) was determined to be 1.00(10) and was found not to depend on the collision energy in the range below kB·10 K. The observed I(N+)/I(N2+) ratio of 1.00(10) corresponds to a branching ratio of 0.50(5) for the two product channels (eqn (2) and (3)). This value is lower than the I(N+)/I(N2+) ratio of about 1.5 observed in most earlier studies (see Table 1). In these studies, the reactions were observed under conditions where part of the N+ yield resulted from the dissociation of metastable N2+ product ions caused by secondary collisions with N2 molecules and He atoms in the background gas, as discussed in, e.g., ref. 39, 42, 53 and 56.These observations indicate that, at low collision energies, about half of the N2+ ions generated in the reaction between He+ and N2 predissociates on a timescale shorter than 1 μs and that the remaining half is formed in longer-lived N2+ states, either in metastable states or in states that radiatively decay to states located below the lowest dissociation threshold of N2+. Two recent studies have examined the predissociation of the different vibrational levels of the C state of N2+.57,70 As explained in the introduction, only v ≤ 4 levels of the C state can be produced at low collision energies and, of these levels, only the v = 3 and 4 levels are located above the lowest dissociation limit of N2+ (see inset of Fig. 1). The predissociation lifetimes of the v = 3 and 4 levels were reported to be 28.7 ns (7.19 ns) and 0.585 ns (2.46 ns) in ref. 57 (ref. 70), respectively. In ref. 57, the radiative lifetime of these levels were reported to be 56.04 and 55.03 ns, respectively. Consequently, 99%, 66% and 0% of the N2+ ions initially generated in the C-state v = 4, 3 and ≤2 levels, respectively, should be detected as N+ ions in our experiment and, correspondingly, 1%, 34% and 100% should be detected as N2+ ions. The fraction of the reactions that initially lead to N2+ ions in the C state is not known. Assuming that the almost resonant charge transfer to the C (v = 4) level dominates over the charge transfer to the C (v ≠ 4) levels and that it is the main source of N+ ions would lead to the conclusion that about 50% of the charge-transfer processes populate rovibrational levels of other electronic states of N2+ that do not predissociate within 1 μs, either because their predissociation lifetimes are longer than 1 μs or because they are longer than their radiative lifetimes.
3.2 Collision-energy dependence of the reaction rate coefficient
Fig. 3(a) displays the sum of the integrated N+ and N2+ ion signals (green circles) measured for different mean velocities (vRyd) of the Rydberg helium beam in the 720–1100 m s−1 range. The error bars are obtained from the statistical analysis of 5 consecutive measurements, each consisting of an average of 500 experimental cycles. The integration windows used to monitor the product ions are indicated in green in Fig. 2. The velocity spread around the mean velocity of the N2 beam (vN2) in the reaction zone is ΔvN2 ≃ ±10 m s−1. The opening of the valve generating the N2 supersonic beam was adjusted for each experimentally selected value of vRyd to ensure that the Rydberg atoms always interact with N2 molecules of the same velocity class. The product-ion signals were corrected for (i) the change of He(n) density resulting from the higher acceleration or deceleration from the initial beam velocity, and (ii) the changing overlap between the packet of He(n) Rydberg atoms and the N2 beam, as discussed in Article I. The data presented in Fig. 3(a) reveal that the product-ion signal gradually decreases as vRyd approaches the velocity vN2 of the N2 beam, which is indicated by the dash-dotted vertical line. The signal reduction is symmetric with respect to vN2 within the error bars.The sum of the N+ and N2+ product-ion signals, I = I(N+) + I(N2+), after correction for the detection bias favouring product yields measured at higher He(n)-beam velocities, is depicted as a function of the collision energy as full green circles in Fig. 3(b). The vertical error bars represent one standard deviation of the five measurements performed at each relative velocity and the horizontal bars correspond to the range of collision energies probed in each case according to eqn (4):
 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ortho-to-para ratio of the nuclear-spin-statistical factors, i.e., 2/3 of the N2 molecules are in states with J = 0, 2, 4… (ortho N2) and 1/3 are in states with J = 1, 3, 5… (para N2).
1 ortho-to-para ratio of the nuclear-spin-statistical factors, i.e., 2/3 of the N2 molecules are in states with J = 0, 2, 4… (ortho N2) and 1/3 are in states with J = 1, 3, 5… (para N2).
The data presented in Fig. 3 indicate that the reaction rate coefficient decreases by 30% as the collision energy Ecoll decreases from kB·10 K to zero. The opposite behaviour, i.e., an enhancement of the reaction rate coefficient with decreasing collision energy, was observed in the charge–quadrupole capture reaction of H2+ with H2 forming H3+ and H.33,71 This difference can be explained by the different sign of the quadrupole tensor element Qzz of N2 and H2, as explained in the next section.
4 The charge–quadrupole interaction and the effect of the sign of the Qzz quadrupole tensor element on the rotational-state-specific capture rate coefficients
The calculations presented in this section follow an adiabatic channel model initially formulated in ref. 11 and 12 for the description of the ion–molecule capture. They disregard the effects of quantum-Langevin scattering near zero collision energy72 and of the Coriolis coupling of adiabatic reaction channels involving rotational states of the neutral molecule of different M values, where M is the quantum number associated with the projection of the rotational angular momentum![[J with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004a_20d1.gif) of the neutral molecule onto the collision axis. These effects were shown to be significant only at very low collision energies (below kB·10 mK) in ref. 15 and 16 and are expected to be negligible at the energy resolution of kB·250 mK of the experiments presented in the previous section.
of the neutral molecule onto the collision axis. These effects were shown to be significant only at very low collision energies (below kB·10 mK) in ref. 15 and 16 and are expected to be negligible at the energy resolution of kB·250 mK of the experiments presented in the previous section.
In the absence of a permanent dipole moment of the neutral molecule, the lowest member of the multipole-moment interaction series between an ion and a neutral molecule is the charge–quadrupole interaction. The simplest molecules without a permanent dipole moment but with a quadrupole moment are homonuclear diatomic molecules (we consider here H2 and 14N2). The quadrupole moment tensor, Q, with elements Qij∈{x,y,z}, describes a distribution of partial charges in the molecular centre-of-mass reference frame. It is a rank-two tensor, usually written as a traceless matrix with only diagonal elements (Qxx, Qyy and Qzz). For symmetric-top and linear molecules, Qxx = Qyy, and the Qzz component of the quadrupole-moment tensor is sufficient to describe the quadrupolar charge distribution.
Classically, the interaction between an ion of charge q and a homonuclear neutral molecule with Qzz component of the quadrupole tensor in the molecular reference frame, can be written as:15,31
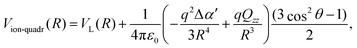 | (5) |
 and θ is the angle between the collision axis and the internuclear axis of the diatomic molecule (see Fig. 5 below). The Langevin interaction potential, VL(R), is given by:
and θ is the angle between the collision axis and the internuclear axis of the diatomic molecule (see Fig. 5 below). The Langevin interaction potential, VL(R), is given by: | (6) |
 and μ are the magnitude of the orbital angular momentum and the reduced mass of the collision, respectively. The second term in eqn (6) is the isotropic component of the charge–induced-dipole interaction, which scales as ∝R−4 and depends on the average polarisability volume of the molecule,
and μ are the magnitude of the orbital angular momentum and the reduced mass of the collision, respectively. The second term in eqn (6) is the isotropic component of the charge–induced-dipole interaction, which scales as ∝R−4 and depends on the average polarisability volume of the molecule,  . The second and third terms in eqn (5) describe the anisotropic components of the interaction between the ion and the molecule as a function of the angle θ. The second term in eqn (5) describes the contribution to the charge–induced-dipole interaction of the polarisability anisotropy
. The second and third terms in eqn (5) describe the anisotropic components of the interaction between the ion and the molecule as a function of the angle θ. The second term in eqn (5) describes the contribution to the charge–induced-dipole interaction of the polarisability anisotropy  , and the third term describes the electrostatic interaction between the charge of the ion and the quadrupolar charge distribution of the molecule, which is proportional to qQzz/R3. The values of the relevant physical quantities in the ground electronic state of N2 and H2 from ref. 31 and 73 are listed in Table 2.
, and the third term describes the electrostatic interaction between the charge of the ion and the quadrupolar charge distribution of the molecule, which is proportional to qQzz/R3. The values of the relevant physical quantities in the ground electronic state of N2 and H2 from ref. 31 and 73 are listed in Table 2.
The quadrupole moment leads to Stark shifts of the molecular rotational level energies in the electric field of the ion. These Stark shifts can be calculated with perturbation theory in the realm of the perturbed-rotor approximation (see, e.g., ref. 15, 30 and 31). Alternatively, one can obtain the exact value of the Stark shifts ΔEJM(R) of the rotational levels |JM〉 by diagonalizing the Hamiltonian:
| Ĥ(R) = Ĥrot + ĤStark(R), | (7) |
 | (8) |
We consider the total charge–quadrupole interaction potential for a molecule in a state which correlates adiabatically to the |JM〉 state in zero electric field as:12,24
| V(JM)int(R) = VL(R) + ΔEJM(R). | (9) |
The calculated interaction potentials between He+ and N2 molecules in rotational states with J ≤ 5 are presented in Fig. 4. They were obtained by diagonalizing the Hamiltonian in eqn (7) in a basis with J ≤ 16 for values of the ion–molecule separation R in the 0.1–60 Å range, in steps of 0.05 Å, and using the data presented in Table 2. The black and coloured lines in Fig. 3 represent the Langevin interaction potential VL and the rotational-state-specific interaction potential V(JM)int(R) for the He+ + N2 reaction, respectively. In each case the full and dashed lines represent the potential obtained for ![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) = 0 and 11, respectively. The charge–quadrupole interaction significantly modifies the interaction potentials associated with all |J, M〉 states and the effect becomes stronger with increasing J value. The interaction potential for the |0, 0〉 state is slightly more attractive than VL because of the field-induced mixing with the |2, 0〉 level. Consequently, the
= 0 and 11, respectively. The charge–quadrupole interaction significantly modifies the interaction potentials associated with all |J, M〉 states and the effect becomes stronger with increasing J value. The interaction potential for the |0, 0〉 state is slightly more attractive than VL because of the field-induced mixing with the |2, 0〉 level. Consequently, the ![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) = 11 centrifugal energy barrier (V
= 11 centrifugal energy barrier (V![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) =11,maxL) in the interaction potential is lowered from kB·1.46 K for a pure Langevin potential to kB·1.01 K. For J ≥ 1, the effect of the charge–quadrupole interaction depends on the value of M. The interaction potentials V(J,M=0)int(R) for M = 0 are more attractive than both VL and V(0,0)int(R) [see Fig. 4(g)] and the effect is strongest for J = 1 and decreases with increasing J value.
=11,maxL) in the interaction potential is lowered from kB·1.46 K for a pure Langevin potential to kB·1.01 K. For J ≥ 1, the effect of the charge–quadrupole interaction depends on the value of M. The interaction potentials V(J,M=0)int(R) for M = 0 are more attractive than both VL and V(0,0)int(R) [see Fig. 4(g)] and the effect is strongest for J = 1 and decreases with increasing J value.
In contrast, the interaction potentials of the states with the maximum value of |M| (i.e., |M| = J) are more repulsive than VL, even for ![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) = 0 head-on collisions, resulting in a potential energy barrier with maxima highlighted by the short coloured horizontal bars in Fig. 4(b–f). The heights of the potential energy barriers increase with the value of J, from V(1,1),
= 0 head-on collisions, resulting in a potential energy barrier with maxima highlighted by the short coloured horizontal bars in Fig. 4(b–f). The heights of the potential energy barriers increase with the value of J, from V(1,1),![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) maxint/kB = 0.250 K for the |1, 1〉 state to V(5,5),
maxint/kB = 0.250 K for the |1, 1〉 state to V(5,5),![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) maxint/kB = 4.894 K for the |5, 5〉 state [see Fig. 4(h)]. For molecules in these states, the reaction is suppressed for collision energies Ecoll < V(J,|M|=J),maxint. For states with M values between these two limiting situations (0 < |M| < J), the interaction potentials V(J,M)int(R) are in general more attractive than VL but less attractive than V(J,0)int(R).
maxint/kB = 4.894 K for the |5, 5〉 state [see Fig. 4(h)]. For molecules in these states, the reaction is suppressed for collision energies Ecoll < V(J,|M|=J),maxint. For states with M values between these two limiting situations (0 < |M| < J), the interaction potentials V(J,M)int(R) are in general more attractive than VL but less attractive than V(J,0)int(R).
The results obtained for the V(J,M)int(R) interaction potentials in the case of the He+ + N2 reaction and displayed in Fig. 4 can be qualitatively understood in terms of electrostatic arguments based on an angular-momentum vector model of the rotation of the diatomic molecule (see Fig. 5). In homonuclear diatomic molecules, a positive (negative) value of the Qzz quadrupole tensor element corresponds to a partial-charge distribution with a partial charge of −2|δ| (+2|δ|) near the molecular centre of mass, and equal, positive (negative) partial charges of + |δ| (−|δ|) near the two ends of the molecule. The negative Qzz value of N2 arises from the excess negative charges from the electron lone pairs on the N atoms. In H2, Qzz is positive because the excess negative charge is located near the molecule geometric centre, where the electron density from the sigma bond is maximal.
 | ||
| Fig. 5 Schematic charge–quadrupole interaction between He+ and N2 in |JM〉 rotational states with M = J (a) and M = 0 (b). | ||
In the angular-momentum vector model (see, e.g., Section 1.4 of the book by Zare74), the rotational angular-momentum vector ![[J with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004a_20d1.gif) of the diatomic molecule precesses around the quantisation axis (which is the collision axis) at an angle given by
of the diatomic molecule precesses around the quantisation axis (which is the collision axis) at an angle given by  . The internuclear axis of a diatomic molecule lies in a plane perpendicular to
. The internuclear axis of a diatomic molecule lies in a plane perpendicular to ![[J with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004a_20d1.gif) . In M = J states, α is minimal and
. In M = J states, α is minimal and ![[J with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004a_20d1.gif) points along the collision axis. Consequently, the molecular rotation takes place in a plane perpendicular to the collision axis, so that the N2 molecule approaches the ion in a “helicoptering” motion [see Fig. 5(a)]. This configuration exposes the region of positive charge to the He+ ion so that the electrostatic interaction is overall repulsive, which explains the increase in the height of the potential barrier compared to the pure Langevin potential. In M = 0 states,
points along the collision axis. Consequently, the molecular rotation takes place in a plane perpendicular to the collision axis, so that the N2 molecule approaches the ion in a “helicoptering” motion [see Fig. 5(a)]. This configuration exposes the region of positive charge to the He+ ion so that the electrostatic interaction is overall repulsive, which explains the increase in the height of the potential barrier compared to the pure Langevin potential. In M = 0 states, ![[J with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004a_20d1.gif) points in a direction perpendicular to the collision axis, so that the N2 rotational motion can be described as a “cartwheeling” motion in the collision plane [see Fig. 5(b)]. In this configuration, the regions of excess negative charges are exposed to the He+ ion and the electrostatic interaction is attractive.
points in a direction perpendicular to the collision axis, so that the N2 rotational motion can be described as a “cartwheeling” motion in the collision plane [see Fig. 5(b)]. In this configuration, the regions of excess negative charges are exposed to the He+ ion and the electrostatic interaction is attractive.
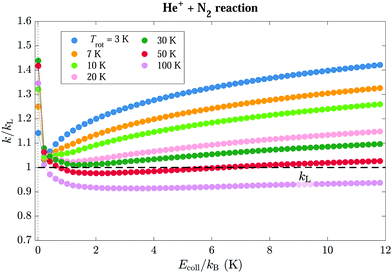
The rotational-state-specific modifications of the interaction potentials caused by the charge–quadrupole interaction has a significant effect on the capture rate coefficients. The state-specific capture rate coefficients for the He+ + N2(J, M) reaction for rotational levels with J ≤ 5 are displayed in colour in Fig. 6(a)–(f) after normalisation to the Langevin rate constant kL represented by the horizontal black lines. The calculations of capture rate coefficients were performed for collision energies in the kB·(0.01–12.01) K range, in steps of 0.2 K, following the procedure described in Article I.17
The rotational states can be classified in four types according to the dependence of the capture rate coefficients on the collision energy. (i) The first group of states contains only the |0, 0〉 state. Its rate coefficient is approximately equal to kL at the lowest energies, and increases with increasing Ecoll, reaching a value of 1.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kL at Ecoll/kB = 12 K. This effect is caused by the mixing between the |0, 0〉 and |2, 0〉 states in the electric field of the ion. (ii) The second group consists of (J, M ≈ 0) states, which exhibit reaction rate coefficients that are overall higher than kL. The rate coefficients are relatively collision-energy-independent for collision energies Ecoll/kB ≳ 2 K, but sharply increase at collision energies below kB·1 K. This effect is most pronounced for the (1, 0) state, which reaches a rate constant of 4.1kL at Ecoll/kB = 0.01 K, and gradually decreases with increasing J value, as can be seen in Fig. 6(g), which compares the rate coefficients of all M = 0 states. (iii) The third group of states comprises the states (J, |M| ≈ J) which have vanishing rate coefficients for collision energies Ecoll ≤ V(J,M),maxint. For these states, the rate coefficients increase with increasing Ecoll beyond Ecoll = V(J,M),maxint [panel (h)]. (iv) The last group of states includes states of relatively high values of J and intermediate values of |M|, such as the |5, ±3〉 and |4, ±3〉 states, for which the interaction potentials do not strongly deviate from the pure Langevin potential, indicating that the role of the charge–quadrupole interaction is minor. These states are characterised by capture rate coefficients that are very close to kL and are almost independent of Ecoll. At high collision energies the rate coefficients of all states converge to the value of kL (see, e.g., left panel of Fig. 9 below for J = 0, 1).
kL at Ecoll/kB = 12 K. This effect is caused by the mixing between the |0, 0〉 and |2, 0〉 states in the electric field of the ion. (ii) The second group consists of (J, M ≈ 0) states, which exhibit reaction rate coefficients that are overall higher than kL. The rate coefficients are relatively collision-energy-independent for collision energies Ecoll/kB ≳ 2 K, but sharply increase at collision energies below kB·1 K. This effect is most pronounced for the (1, 0) state, which reaches a rate constant of 4.1kL at Ecoll/kB = 0.01 K, and gradually decreases with increasing J value, as can be seen in Fig. 6(g), which compares the rate coefficients of all M = 0 states. (iii) The third group of states comprises the states (J, |M| ≈ J) which have vanishing rate coefficients for collision energies Ecoll ≤ V(J,M),maxint. For these states, the rate coefficients increase with increasing Ecoll beyond Ecoll = V(J,M),maxint [panel (h)]. (iv) The last group of states includes states of relatively high values of J and intermediate values of |M|, such as the |5, ±3〉 and |4, ±3〉 states, for which the interaction potentials do not strongly deviate from the pure Langevin potential, indicating that the role of the charge–quadrupole interaction is minor. These states are characterised by capture rate coefficients that are very close to kL and are almost independent of Ecoll. At high collision energies the rate coefficients of all states converge to the value of kL (see, e.g., left panel of Fig. 9 below for J = 0, 1).
To highlight the effect of the sign of Qzz on the rate coefficients, calculations for the D2+ + H2 ion–molecule reaction were performed following the same procedure as for the He+ + N2 reaction [the He+ + H2 reaction is a slow reaction that does not follow Langevin behaviour, see, e.g., ref. 75. We therefore replaced He+ by D2+ because of their almost equal mass]. H2 has a positive quadrupole moment of Qzz = 0.638 D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Å
Å![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 and a rotational constant of B0(H2) = 59.3398 cm−1,73 more than an order of magnitude larger than B0(N2). The relevant interaction potentials are presented in Fig. 7 and the rotational-state-specific reaction rate coefficients are depicted in Fig. 8.
31 and a rotational constant of B0(H2) = 59.3398 cm−1,73 more than an order of magnitude larger than B0(N2). The relevant interaction potentials are presented in Fig. 7 and the rotational-state-specific reaction rate coefficients are depicted in Fig. 8.
Because of the positive value of Qzz in the case of H2, the states with M = 0 (|M| = J) are now the states that experience a repulsive (attractive) electrostatic interaction, as can be immediately understood from the considerations based on the vector model (see Fig. 5). An approach in the helicoptering (|M| = J) configuration indeed now exposes the negative partial charge near the molecule geometric centre and an approach in the cartwheeling configuration exposes to the ion the positive partial charges at the ends of the molecule. Attractive and repulsive contributions to the interaction potential result, respectively. Consequently, the behaviour of the states in categories (ii) and (iii) is exchanged when N2 is replaced by H2. In contrast to the situation encountered in N2, however, the height of the potential energy barrier for M = 0 states, V(J,0),maxint, decreases with increasing J. This difference is the result of the larger rotational constant in H2, which increases the energetic separation between adjacent J levels and reduces the state mixing caused by the charge–quadrupole interaction. For the same reason, the interaction potentials involving |M| = J states become more attractive for increasing J value. The deviation of the V(0,0)int(R) potential from VL in H2 is also strongly reduced compared to the situation encountered in N2 because the larger rotational constant in H2 leads to a much reduced mixing between the |0, 0〉 and |2, 0〉 states.
The rotational-state-dependent capture rate constants for the D2+ + H2 reaction displayed in Fig. 8 reflect these considerations. Because the |0, 0〉 state hardly gets mixed with the |2, 0〉 state, its rate coefficient is almost Ecoll – independent and does not significantly deviate from kL [Fig. 8(a)]. It weakly increases with increasing Ecoll and reaches a value of 1.02kL at Ecoll/kB = 12 K. The rate-coefficient enhancement of the |M| = J states at the lowest collision energies increases with the J value [see panel Fig. 8(h)], reaching a value of 3.49kL for the (5,5) state at 0.01 K. This effect can be understood in terms of the reduced precession angle α and the resulting energetically more favourable configuration for H2 [see Fig. 5(a)].
The rate coefficients associated with M = 0 states of H2 [Fig. 8(g)] increase faster with increasing Ecoll than those associated with |M| = J states of N2, because for these states the potential-energy barriers are smaller in H2 than in N2 [compare Fig. 8(g) with Fig. 6(h)]. A second important difference between the reactions of He+ with N2 and D2+ with H2 concerns the behaviour of the J-dependent capture rate coefficients after averaging over the M states, as depicted for J = 0, 1, and 2 in Fig. 6(i) for N2 and in Fig. 8(i) for H2. At the lowest collision energies, the rate coefficients for the J = 1 and 2 states are highest but they rapidly decrease as the collision energy increases and then start growing again. In the case of N2, the rate coefficients become larger than kL and reach a maximum at collision energies in the range between kB·50 K and kB·100 K, before decreasing again towards kL at high collision energies, see left panel of Fig. 9. In the case of H2, the rates do not significantly increase beyond kL and remain constant at collision energies beyond kB·10 K, see Fig. 8(i) and right panel of Fig. 9.
The collision-energy dependence up to kB·106 K of the rate coefficients involving the J = 0 and 1 rotational levels of N2 and H2 are compared in Fig. 9. These levels are the ground states of the two nuclear-spin isomers and have nuclear-spin statistical factors of 1 (J = 0, para) and 3 (J = 1, ortho) in H2, and 6 (J = 0, ortho) and 3 (J = 1, para) in N2. The main difference in behaviour concerns the J = 1 levels and originates from the opposite sign of the quadrupole tensor element Qzz, as discussed above. The charge–quadrupole interaction is attractive for M = 0 in N2 and for |M| = 1 in H2, which leads to an enhancement of the rate coefficients compared to kL at the lowest collision energies. In contrast, the interaction is repulsive for |M| = 1 in N2 and M = 0 in H2, which leads to the complete suppression of the reaction rates. The second difference originates from the mixing of rotational levels induced by the field of the ion, which induces a red Stark shift of the lowest rotational levels (J = 0, 1) with M = 0 in N2 and |M| = 1 in H2 and an increase of the rate coefficients compared to kL. In N2, this effect is twice stronger than in H2 because the rotational levels are much more closely spaced.
States with |M| > 0 and M = 0 have degeneracy factors of 2 and 1, respectively, and J ≥ 1, M = 0 (|M| = 1) states have attractive (repulsive) potentials in H2 and repulsive (attractive) potentials in N2. Consequently, the overall dependence of the rate coefficients on the collision energy after averaging over the M sublevels is different. In H2, the kJ=1 capture rate coefficient only slightly decreases with decreasing energy, reaching a minimum of approximately 0.95kL at Ecoll/kB = 1.55 K [see inset of Fig. 8(i)] before increasing again to a value of 1.6kL at 10 mK. In N2, the decrease of the rate coefficient below kB·10 K is more pronounced and amounts to about 30%. This behaviour reflects the fact that rate coefficients of the M = ±1 states become zero when the collision energy drops below the barrier in the interaction potential. It is only at extremely low collision energies, below kB·50 mK, that the effect of the rate coefficient of the M = 0 state becomes dominant again, causing the M-averaged rate coefficient to rise again and reach a value of 1.35kL at kB·10 mK.
5 Discussion
5.1 Comparison with experimental results
In the case of the ion–molecule reactions involving H2 and D2, the validity of the adiabatic capture model had already been verified prior to this work by comparing the results of the calculations of Dashevskaya et al.16 with experimental results.33,71For comparison with the experimental data reported in Section 3 for the He+ + N2 reaction, the rotational-state-specific and collision-energy-dependent rate coefficients must be averaged to account for the finite energy resolution of the measurements and for the fact that several rotational states of N2 are populated in the supersonic expansion. In the range of collision energies probed experimentally (Ecoll/kB between 0 and 12 K), the averaged capture rate coefficients depend strongly on the rotational temperature Trot of the N2 sample in the supersonic beam. This dependence is illustrated in Fig. 10 which displays the collision-energy-dependent rate coefficients k(Ecoll) after averaging over the rotational-state-population distribution according to
 | (10) |
At the rotational temperature of 7.0 K, the average rate coefficient k(Ecoll) decreases by more than 30% from a value of about 1.5 kL at Ecoll/kB = 12 K to ≈kL at Ecoll/kB ≈ 0.2 K before growing again at the lowest collision energies, as explained in the previous section. At higher rotational temperatures (Trot ≥ 20 K), k(Ecoll) becomes much less dependent on the collision energy and its value gradually decreases in the collision-energy range investigated experimentally.
At the rotational temperature of 7.0 ± 1.0 K determined experimentally (see Section 2), only the J = 0, 1, and 2 levels of N2 are significantly populated [see inset of Fig. 3(b)]. A comparison of the calculated rate coefficients presented in Fig. 10 with the experimental data in Fig. 3(b) shows qualitative agreement for rotational temperatures below 10 K. The sharp rise of the calculated rate coefficients near zero collision energies is indeed not observable experimentally because of the limited collision-energy resolution. A quantitative analysis requires averaging over the distribution of collision energies given by eqn (4). The dependence of k(Ecoll) on Trot is large. Optimizing the value of Trot to best reproduce the experimentally observed rate coefficients led to the same value for Trot (7.0 ± 1.0 K) as obtained by 2 + 1 REMPI spectroscopy (see Section 2). The result of the quantitative analysis are presented with the experimental data in Fig. 3(b). The violet circles in Fig. 3(b) are weighted sums of the calculated rotational-state-dependent reaction rate coefficients (see Section 4), for a rotational temperature of Trot = 7.0 K, which was found to best describe the experimental data. The inset displays the corresponding rotational population distribution of N2, including nuclear-spin-statistics effects. The solid black line depicts the Gaussian convolution of the calculated weighed sum of the rotational-state-dependent rate coefficients taking into account the experimental energy resolution, as described in ref. 26. This curve reproduces the experimental data well. Given that the analysis does not contain any adjustable parameter other than Trot and the collision-energy resolution at the lowest collision energies [ΔEres in eqn (4)], we conclude that the adiabatic capture model presented in Section 4 is adequate to describe the capture rate coefficient at low collision energies, where the charge–quadrupole interaction plays an important role.
Rowe et al.9 have measured the thermal rate constant of the He+ + N2 reaction at temperatures of 8, 20 and 28 K in uniform supersonic flows (CRESU technique). To compare with these experimental results, thermal capture rate constants were determined from the collision-energy-dependent rotational-state-specific rate coefficients presented in Section 4 using eqn (20) and (21) of Article I.17 The comparison is presented in Fig. 11, which depicts the experimental results of ref. 9 as violet squares with error bars and the calculated thermal rate constants as black open circles and full line. The calculated capture rates are larger than the measured reaction rates, which suggests that not all capture processes lead to a reaction. If one assumes that the discrepancy between the calculated and measured reaction rate constants originates from a less-than-unity reaction probability upon close encounter of the reactants and that the reaction probability is independent of the collision energy, the reaction probability can be estimated by scaling down the calculated thermal capture rate constants until they match the experimental data (see orange open circles and full line). This procedure suggests that the reaction probability is 0.65(20). Unfortunately, the experimental error bars are too large for any conclusion to be drawn concerning the predicted temperature dependence of the thermal rate constant.
 | ||
| Fig. 11 Comparison of the calculated thermal capture rate constant for the He+ + N2 reaction (black line and open circles) with the Langevin rate (dashed horizontal red line) and the thermal rate constants measured by Rowe et al.9 at temperatures of 8, 20 and 28 K using the CRESU technique (violet squares with error bars). The orange open circles and line represent the calculated rate constants scaled by a factor of 0.625. | ||
5.2 Comparison with earlier calculations
Troe and coworkers have reported detailed calculations of the capture rate coefficients of ion–molecule reactions involving molecules having an electric quadrupole moment.15,16,30,31 These calculations introduced and tested several approximations in the treatment of the interaction potentials and served as reference to validate our results. In contrast to the present calculations, the calculations of Dashevskaya et al.15,16,31 also considered the effects of quantum scattering and of the Coriolis interaction, which mixes rotational levels of the neutral molecule of different M values. These effects were found to lead to (i) an increase of the kJ=0 capture rate coefficients to a value of 2kL at very low collision energies (below kB·0.3 mK for the He+ + N2 reaction), regardless of the sign of the qQzz product, and (ii) a suppression of the divergence of the kJ=1 capture rate coefficients at very low temperatures, such that the kJ=1,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He++N2 (kJ=1,
He++N2 (kJ=1,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar++H2) rate coefficient averaged over all M states stabilises to a finite value at the lowest collision energies ∼kB·10 nK, i.e., to ∼11kL (∼5.6kL). As explained in ref. 15, these effects only become significant at extremely low collision energies, and they are not detectable at the collision energy resolution of ∼kB·250 mK of our experiments (see Section 2) and were therefore ignored in our calculations.
Ar++H2) rate coefficient averaged over all M states stabilises to a finite value at the lowest collision energies ∼kB·10 nK, i.e., to ∼11kL (∼5.6kL). As explained in ref. 15, these effects only become significant at extremely low collision energies, and they are not detectable at the collision energy resolution of ∼kB·250 mK of our experiments (see Section 2) and were therefore ignored in our calculations.
The calculated capture rate coefficients presented in the previous section are consistent with the calculations of Dashevskaya et al.,15 in which the perturbed-rotor (PR) approximation was used to calculate the Stark shifts of the rotational levels of the neutral molecule. In the case of the capture of H2 by an ion, the PR approximation accurately describes the Stark shifts of the |1, 0〉 and |1, 1〉 rotational levels and the kH2(1,0) and kH2(1,1) capture rate coefficients. A small difference between the PR approximation and the exact calculation is observed for the |1, 0〉 level. In the PR approximation, the kH2(0,0) rate coefficient is predicted to be exactly equal to kL over the temperature range considered, whereas the exact treatment through diagonalisation of the Stark Hamiltonian leads to a small (about 2%) increase of kH2(0,0) as Ecoll increases up to kB·12 K [see inset of Fig. 8(a)]. For reactions involving N2, the kN2(0,0) and kN2(1,0) rate coefficients are accurately predicted using the perturbed-rotor approximation, but deviations from the rate coefficients calculated with the exact method are apparent for the (1, 1) state, with  (Ecoll/kB = 12 K) ≈ 0.5
(Ecoll/kB = 12 K) ≈ 0.5 (Ecoll/kB = 12 K).
(Ecoll/kB = 12 K).
6 Conclusions
We have presented the first experimental measurements of the N+ and N2+ product yields of the He+ + N2 reaction for a range of collision energies between 0 and kB·10 K. The reaction was studied within the orbit of the helium Rydberg electron. The product-ions ratio was determined to be N+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2+ ≈ 0.50(5)
N2+ ≈ 0.50(5)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50(5), whereas most previous studies had shown a consistent bias towards the generation of N+, with N+
0.50(5), whereas most previous studies had shown a consistent bias towards the generation of N+, with N+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2+ ≈ 0.60
N2+ ≈ 0.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.40.37,41,50–52,55 We attribute the higher fraction of N2+ ions observed in our experiments to the absence of collisional dissociation in the supersonic beams during the short reaction-observation time of 7 μs. The dissociation of metastable N2+ molecules into an N+ ion and an N atom induced by secondary collisions has been observed in earlier experiments carried out in the presence of higher background gas densities.42,53,56
0.40.37,41,50–52,55 We attribute the higher fraction of N2+ ions observed in our experiments to the absence of collisional dissociation in the supersonic beams during the short reaction-observation time of 7 μs. The dissociation of metastable N2+ molecules into an N+ ion and an N atom induced by secondary collisions has been observed in earlier experiments carried out in the presence of higher background gas densities.42,53,56
A ≈30% decrease of the reaction yield was observed when the collision energy was decreased from kB·10 K to 0. To explain this observation, calculations of the rotational-state-specific capture rate coefficients were performed using a statistical adiabatic-channel model inspired by earlier works11,12 and explicitly calculating the Stark shifts of the rotational levels of the neutral molecule in the field of the ion. The excellent agreement obtained between the calculated and observed energy dependence of the rate coefficients [see Fig. 3(b)] indicates that the treatment of the charge–quadrupole interaction through diagonalisation of the Stark Hamiltonian in the realm of the adiabatic channel model leads to reliable interaction potentials and capture rate coefficients in the range of collision energies investigated experimentally. The comparison of experimental and theoretical results for the He+ + N2 reaction and with results obtained on ion–molecule reactions involving H2 molecules enabled the first clear observation and quantification of the role of the sign of the quadrupole moment of the neutral molecule in the capture rate coefficients of ion–molecule reactions. At low collision energies, the capture rate coefficients of ion–molecule reactions characterised by a positive (negative) qQzz product were found to increase (decrease) with decreasing collision energy.
For low J values and intermediate collision energies, i.e., in the range beyond kB·10 K (see Fig. 9), quadrupolar molecules with a small rotational constant, such as N2, were found to have rate coefficients that are larger than the Langevin rate constant kL, whereas molecules with a large rotational constant, such as H2, have capture rate coefficients that are much closer to kL. This behaviour can be explained by the fact that in quadrupolar molecules with small rotational constants the Stark shifts originating from the interaction between the electric quadrupole and the field of the ion are stronger than in molecules with large rotational constants. The nonlinear Stark shifts become significant at much lower fields and are on the average negative, which effectively reduces the heights of the centrifugal barriers compared to the pure Langevin case and increases the rate coefficients. The calculations also indicate that the deviations from Langevin capture behaviour rapidly decrease with increasing collision energy and rotational excitation of the neutral molecule.
The comparison of calculated thermal capture rate coefficients for the He+ + N2 reactions with thermal reaction rate constants measured in uniform supersonic flow9 suggests that the probability of reaction upon close encounter of He+ and N2 is less than unity, probably in the range 0.63 ± 0.20.
Calculations of the rotational-state-specific and collision-energy-dependent rate coefficients of ion–molecule reactions involving H2 and N2 revealed a large sensitivity on the absolute value of the magnetic rotational quantum number M of the neutral molecule. Moreover, changing the sign of the quadrupole was found to exchange the role played by rotational states with M = 0 and |M| = J. This behaviour was explained qualitatively by simple electrostatic considerations based on the angular-momentum vector model (see Fig. 5).
The calculations reveal that a rich behaviour could be explored in experiments using neutral-molecule samples prepared in states with aligned rotational angular momentum. In the future, efforts should also be invested to further improve the collision-energy resolution of the experiments, with the prospect of observing the effects of quantum scattering and Coriolis coupling predicted in ref. 15 and 16. These effects decrease rapidly with increasing reduced mass of the collision system. In this respect, the H2+ + H2 reaction (μ ≈ 1 u) offers advantages over the He+ + N2 reaction (μ ≈ 3.5 u) and work on the H2+ + H2 reaction is currently under way in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Joel Jenny for his contributions to the data acquisition, Katharina Höveler and Johannes Deiglmayr for fruitful discussions, Josef A. Agner and Hansjürg Schmutz for technical assistance, and Majdi Hochlaf and Laurent Nahon for making the potential-energy functions of N2+ depicted in Fig. 1 available in digital form. This work is supported financially by the Swiss National Science Foundation (Grant No. 200020B-200478) and by the European Research Council through the ERC advanced grant (Grant No. 743121) under the European Union's Horizon 2020 research and innovation programme.References
- S. Willitsch, Chemistry with Controlled Ions, Advances in Chemical Physics, ed. S. A. Rice and A. R. Dinner, 2017, vol. 162, pp. 307–340 Search PubMed.
- B. R. Heazlewood and T. P. Softley, Nat. Rev. Chem., 2021, 5, 125–140 CrossRef CAS.
- L. Ploenes, P. Straňák, H. Gao, J. Küpper and S. Willitsch, Mol. Phys., 2021, 119, e1965234 Search PubMed.
- I. Savić, S. Schlemmer and D. Gerlich, Astrophys. J., 2005, 621, 1163–1170 CrossRef.
- D. Gerlich, The study of cold collisions using ion guides and traps, in Low Temperatures and Cold Molecules, ed. I. M. W. Smith, Imperial College Press, London, 2008, ch. 3, pp. 121–174 Search PubMed.
- R. Wester, J. Phys. B: At., Mol. Opt. Phys., 2009, 42, 154001 CrossRef.
- C. Markus, O. Asvany, T. Salomon, P. Schmid, S. Brünken, F. Lipparini, J. Gauss and S. Schlemmer, Phys. Rev. Lett., 2020, 124, 233401 CrossRef CAS PubMed.
- J. B. Marquette, B. R. Rowe, G. Dupeyrat, G. Poissant and C. Rebrion, Chem. Phys. Lett., 1985, 122, 431–435 CrossRef CAS.
- B. R. Rowe, J. B. Marquette, G. Dupeyrat and E. E. Ferguson, Chem. Phys. Lett., 1985, 113, 403–406 CrossRef CAS.
- B. Rowe, A. Canosa and V. L. Page, Int. J. Mass Spectrom. Ion Processes, 1995, 149, 573–596 CrossRef.
- D. C. Clary, Mol. Phys., 1985, 54, 605–618 CrossRef CAS.
- J. Troe, Chem. Phys., 1987, 87, 2773–2780 CAS.
- M. Auzinsh, E. I. Dashevskaya, I. Litvin, E. E. Nikitin and J. Troe, J. Chem. Phys., 2013, 139, 084311 CrossRef CAS PubMed.
- M. Auzinsh, E. I. Dashevskaya, I. Litvin, E. E. Nikitin and J. Troe, J. Chem. Phys., 2013, 139, 144315 CrossRef CAS PubMed.
- E. I. Dashevskaya, I. Litvin, E. E. Nikitin and J. Troe, J. Chem. Phys., 2005, 122, 184311 CrossRef CAS PubMed.
- E. I. Dashevskaya, I. Litvin, E. E. Nikitin and J. Troe, J. Chem. Phys., 2016, 145, 244315 CrossRef CAS.
- V. Zhelyazkova, F. B. V. Martins, J. A. Agner, H. Schmutz and F. Merkt, Phys. Chem. Chem. Phys., 2021, 23, 21606–21622 RSC.
- V. Zhelyazkova, F. B. V. Martins and F. Merkt, submitted for publication.
- D. Albritton, At. Data Nucl. Data Tables, 1978, 22, 1–89 CrossRef CAS.
- V. G. Anicich and W. T. Huntress, Astrophys. J. Suppl. Ser., 1986, 62, 553–672 CrossRef CAS.
- Kinetics of Ion-Molecule Reactions, ed. P. Ausloos, Plenum Press, New York, 1978 Search PubMed.
- Gas Phase Ion Chemistry, ed. M. T. Bowers, Academic Press, New York, 1979, vol. 1 and 2 Search PubMed.
- State-selected and state-to-state ion-molecule reaction dynamics: Part 1. Experiment and Part 2. Theory, ed. C.-Y. Ng and M. Baer, John Wiley & Sons, Inc., New York, 1992 Search PubMed.
- D. C. Clary, Annu. Rev. Phys. Chem., 1990, 41, 61–90 CrossRef CAS.
- J. Troe, J. Chem. Phys., 1996, 105, 6249–6262 CrossRef CAS.
- V. Zhelyazkova, F. B. V. Martins, J. A. Agner, H. Schmutz and F. Merkt, Phys. Rev. Lett., 2020, 125, 263401 CrossRef CAS PubMed.
- T. Stoecklin, D. C. Clary and A. Palma, J. Chem. Soc., Faraday Trans., 1992, 88, 901–908 RSC.
- M. T. Bell, A. D. Gingell, J. M. Oldham, T. P. Softley and S. Willitsch, Faraday Discuss., 2009, 142, 73–91 RSC.
- A. Kilaj, H. Gao, D. Rösch, U. Rivero, J. Küpper and S. Willitsch, Nat. Commun., 2018, 9, 2096 CrossRef PubMed.
- S. C. Smith and J. Troe, J. Chem. Phys., 1992, 97, 5451–5464 CrossRef CAS.
- E. I. Dashevskaya, I. Litvin, E. E. Nikitin and J. Troe, J. Chem. Phys., 2004, 120, 9989–9997 CrossRef CAS PubMed.
- C. Graham, D. A. Imrie and R. E. Raab, Mol. Phys., 1998, 93, 49–56 CrossRef CAS.
- P. Allmendinger, J. Deiglmayr, K. Höveler, O. Schullian and F. Merkt, J. Chem. Phys., 2016, 145, 244316 CrossRef PubMed.
- K. Höveler, J. Deiglmayr, J. A. Agner, H. Schmutz and F. Merkt, Phys. Chem. Chem. Phys., 2021, 23, 2676–2685 RSC.
- P. Warneck, J. Chem. Phys., 1967, 47, 4279–4281 CrossRef CAS.
- D. B. Dunkin, F. C. Fehsenfeld, A. L. Schmeltekopf and E. E. Ferguson, J. Chem. Phys., 1968, 49, 1365–1371 CrossRef CAS.
- A. L. Schmeltekopf, E. E. Ferguson and F. C. Fehsenfeld, J. Chem. Phys., 1968, 48, 2966–2973 CrossRef CAS.
- W. Lindinger, F. C. Fehsenfeld, A. L. Schmeltekopf and E. E. Ferguson, J. Geophys. Res., 1974, 79, 4753–4756 CrossRef.
- T. R. Govers, F. C. Fehsenfeld, D. L. Albritton, P. G. Fournier and J. Fournier, Chem. Phys. Lett., 1974, 26, 134 CrossRef CAS.
- T. Govers, C. van de Runstraat and F. de Heer, Chem. Phys., 1975, 9, 285–299 CrossRef CAS.
- N. G. Adams and D. Smith, J. Phys. B: At. Mol. Phys., 1976, 9, 1439–1451 CrossRef CAS.
- T. R. Govers, M. Gérard, G. Mauclaire and R. Marx, Chem. Phys., 1977, 23, 411–427 CrossRef CAS.
- H. Sekiya, M. Tsuji and Y. Nishimura, J. Chem. Phys., 1987, 87, 325–330 CrossRef CAS.
- P. R. Kemper and M. T. Bowers, J. Chem. Phys., 1973, 59, 4915 CrossRef CAS.
- W. B. Maier, Planet. Space Sci., 1968, 16, 477–493 CrossRef CAS.
- S. J. Bauer, J. Geophys. Res., 1966, 71, 1508–1511 CrossRef CAS.
- O. Dutuit, N. Carrasco, R. Thissen, V. Vuitton, C. Alcaraz, P. Pernot, N. Balucani, P. Casavecchia, A. Canosa, S. L. Picard, J.-C. Loison, Z. Herman, J. Zabka, D. Ascenzi, P. Tosi, P. Franceschi, S. D. Price and P. Lavvas, Astrophys. J. Suppl. Ser., 2013, 204, 20 CrossRef.
- M. Womack, L. M. Ziurys and S. Wyckoff, Astrophys. J., 1992, 393, 188 CrossRef CAS.
- D. Smith, Chem. Rev., 1992, 92, 1473–1485 CrossRef CAS.
- J. Heimerl, R. Johnsen and M. A. Biondi, J. Chem. Phys., 1969, 51, 5041–5048 CrossRef CAS.
- V. G. Anicich, J. B. Laudenslager, W. T. Huntress and J. H. Futrell, J. Chem. Phys., 1977, 67, 4340–4350 CrossRef CAS.
- J. B. Laudenslager, W. T. Huntress and M. T. Bowers, J. Chem. Phys., 1974, 61, 4600–4617 CrossRef CAS.
- R. Marx, G. Mauclaire and S. Fenistein, Chem. Phys. Lett., 1975, 33, 357–361 CrossRef CAS.
- W. Lindinger, E. Alge, H. Störi, R. Varney, H. Helm, P. Holzmann and M. Pahl, Int. J. Mass Spectrom. Ion Phys., 1979, 30, 251–261 CrossRef CAS.
- A. L. Farragher, Trans. Faraday Soc., 1970, 66, 1411 RSC.
- P. R. Kemper and M. T. Bowers, Chem. Phys. Lett., 1975, 36, 183 CrossRef CAS.
- T. Ayari, M. Desouter-Lecomte, R. Linguerri, G. A. Garcia, L. Nahon, A. B. Houria, H. Ghalila, R. B. Said and M. Hochlaf, Adv. Phys.: X, 2020, 5, 1831955 CAS.
- A. Kramida, Y. Ralchenko, J. Reader and N. A. Team, NIST Atomic Spectra Database (ver. 5.8), available: https://physics.nist.gov/asd, National Institute of Standards and Technology, Gaithersburg, MD, 2021 Search PubMed.
- R. Seiler, U. Hollenstein, G. M. Greetham and F. Merkt, Chem. Phys. Lett., 2001, 350, 189 CrossRef CAS.
- X. Tang, Y. Hou, C. Y. Ng and B. Ruscic, J. Chem. Phys., 2005, 123, 074330 CrossRef PubMed.
- F. Merkt and P. M. Guyon, J. Chem. Phys., 1993, 99, 3400–3410 CrossRef CAS.
- X. Li and J. Paldus, J. Chem. Phys., 2008, 129, 054104 CrossRef PubMed.
- C. A. van de Runstraat, F. J. de Heer and T. R. Govers, Chem. Phys., 1974, 3, 431–450 CrossRef CAS.
- J. C. Lorquet and M. Desouter, Chem. Phys. Lett., 1972, 16, 136 CrossRef CAS.
- P. Erman, Phys. Scr., 1976, 14, 51–54 CrossRef CAS.
- A. Lofthus and P. H. Krupenie, J. Phys. Chem. Ref. Data, 1977, 6, 113–307 CrossRef CAS.
- P. Allmendinger, J. Deiglmayr, J. A. Agner, H. Schmutz and F. Merkt, Phys. Rev. A: At., Mol., Opt. Phys., 2014, 90, 043403 CrossRef.
- S. R. Mackenzie, F. Merkt, E. J. Halse and T. P. Softley, Mol. Phys., 1995, 86, 1283–1297 CrossRef CAS.
- F. B. V. Martins, V. Zhelyazkova, Ch. Seiler and F. Merkt, New J. Phys., 2021, 23, 095011 CrossRef CAS.
- B. Paulus, J. F. Pérez-Torres and C. Stemmle, Phys. Rev. A, 2016, 94, 053423 CrossRef.
- K. Höveler, J. Deiglmayr and F. Merkt, Mol. Phys., 2021, 119, e1954708 CrossRef.
- E. Vogt and G. H. Wannier, Phys. Rev., 1954, 95, 1190–1198 CrossRef CAS.
- K. P. Huber and G. Herzberg, Molecular spectra and molecular structure; Volume IV – Constants of diatomic molecules, Van Nostrand Reinhold Company, New York, 1979 Search PubMed.
- R. N. Zare, Angular Momentum, John Wiley & Sons, New York, 1988 Search PubMed.
- M. M. Schauer, S. R. Jefferts, S. E. Barlow and G. H. Dunn, J. Chem. Phys., 1989, 91, 4593–4596 CrossRef CAS.
| This journal is © the Owner Societies 2022 |






![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) = 0 and 11 are indicated by full and dashed lines, respectively. Panels (a)–(f) present the results for |
= 0 and 11 are indicated by full and dashed lines, respectively. Panels (a)–(f) present the results for |