NP monolayer supported transition-metal single atoms for electrochemical water splitting: a theoretical study†
Abstract
The development of cost-effective and highly efficient electrocatalysts for water splitting is highly desirable but remains an ongoing challenge. Numerous single-atom catalysts (SACs) have achieved satisfactory performances in this area; however, non-carbon metal-free substrates have been rarely explored. Herein, we report a series of single-metal atoms supported on a novel two-dimensional NP monolayer as promising electrocatalysts for the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) by theoretical calculations. Our results disclose that Ti@NP, V@NP and Ir@NP exhibit desirable catalytic activity for the HER with extremely low 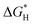 of −0.004, −0.051, and 0.017 eV, respectively. More importantly, the calculated activation barriers for the Tafel reactions of these SACs are much lower than those for the benchmark Pt catalysts. In addition, Pt@NP shows the lowest ηOER of 0.495 V, followed by Rh@NP (ηOER = 0.548 V), which are even superior to that of state-of-the-art IrO2. This work highlights the potential application of metal-free supports in SACs, which also further enriches the application of a NP monolayer in other related electrochemical processes.
of −0.004, −0.051, and 0.017 eV, respectively. More importantly, the calculated activation barriers for the Tafel reactions of these SACs are much lower than those for the benchmark Pt catalysts. In addition, Pt@NP shows the lowest ηOER of 0.495 V, followed by Rh@NP (ηOER = 0.548 V), which are even superior to that of state-of-the-art IrO2. This work highlights the potential application of metal-free supports in SACs, which also further enriches the application of a NP monolayer in other related electrochemical processes.



 Please wait while we load your content...
Please wait while we load your content...