The mechanism of semiconductor to metal transition in the hydrogenation of VO2: a density functional theory study
Abstract
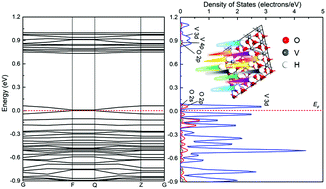
VO2 is a glamorous material with specific metal–semiconductor-transition (MST). The hydrogenation of VO2 could make it a promising material for application in an ambient environment. In this work, we reveal the hydrogenation of VO2 by modulating the hydrogen content and monitoring interaction models, adsorption energies, the density of states, electron density, and charge transfer between hydrogen and the VO2 surface. The monoclinic VO2(020) surface shows a distinct electronic polarization, and the majority spin band gap is larger than the minority spin band gap. The energy gap of the monoclinic VO2 surface is highly dependent on the majority spin band. The interaction between hydrogen and oxygen in the first layer of the VO2 surface is stronger than those in other layers. The energy gap on the surface of VO2 decreases gradually with increasing hydrogen content, and when twelve hydrogen atoms are adsorbed on the surface, an energy gap of 0 eV eventually appears, suggesting that monoclinic VO2 turns into a metallic conductor from a semiconductor. In the process of VO2 hydrogenation, the electron transfer only occurs between hydrogen and its connected oxygen atoms on the VO2 surface, and vanadium atoms just play an intermediary role.


 Please wait while we load your content...
Please wait while we load your content...