 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The solid-state hierarchy and iodination potential of [bis(3-acetaminopyridine)iodine(I)]PF6†
Jas S.
Ward
 *
*
Department of Chemistry, University of Jyvaskyla, Jyväskylä 40014, Finland. E-mail: james.s.ward@jyu.fi
First published on 30th September 2022
Abstract
The first iodine(I) complex bearing hydrogen-bond donor and acceptor groups, [bis(3-acetaminopyridine)iodine(I)]PF6 (3), was synthesised, which exhibited two temperature-dependent solid-state connectivities of the hydrogen bonding. Upon reaction of 3 with tBuOMe, unprecedented iodination of a tBu methyl group proceeded under exceptionally mild conditions in good yield.
Halogen bonding has rapidly matured into one of the most studied intermolecular interactions, and comparisons can be drawn with hydrogen bonding. Hydrogen-bonded networks have a well-established history of practical uses, from body armour to constituting the double helix of our DNA, and whilst not as ubiquitous, the advantages of halogen bonding, like a strictly enforced linear geometry due to its electronic origins as a σ-hole interaction,1 have been deftly utilised toward constructing supramolecular architectures via self-assembling methodologies.2,3 The subfield of halogen bonding incorporating formally cationic halogen atoms, X+ (X = Cl, Br, I), termed halogen(I) ions has been known since the 1960s,4 but was popularised by Barluenga in the 1990s due to the myriad of organic transformations they can perform.5–7 The closely related family of carbonyl or alkyl hypoiodites (R–OI; R = carbonyl or alkyl), recently demonstrated for the first time to be comparable to halogen-bonded iodine(I) species,8,9 are often utilised as in situ generated reagents.10,11 Alkyl hypoiodites such as “tBuOI”,12,13 for which the exact reactive species is still under debate,14 have been reported to perform difficult synthetic conversions such as the synthesis of neopentyl iodide from unactivated neopentane (Scheme 1).15 However, iodinations are predominantly limited to substrates which possess sacrificial functional groups (e.g., hydroxyl or carboxylic acids),16–18 require exotic reagents or methodologies,18–20 and entail multi-step syntheses.21,22 Given that the formation of C–I functionalities remains non-trivial, this creates a void in the literature that iodine(I) species might be able to fill with further research, especially as C–I compounds are of great utility and can act as feedstocks in a wide range of synthetic procedures such as Pd-cross coupling reactions.23–25
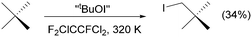 | ||
| Scheme 1 The synthesis of neopentyl iodide from unactivated neopentane using the in situ generated iodine(I) reagent “tBuOI”. | ||
The concept of hierarchical materials, that is materials with structural elements which themselves have structure,26 has found success in a variety of fields including bio-inspired and biomimetic materials,27,28 as well as toward materials for medical applications.29,30 To date, examples of iodine(I) complexes in the solid-state incorporate pyridine,31 or pyridine derivatives with no secondary functional groups capable of meaningful intermolecular interactions,32,33 with only a handful of examples of a single ligand, 4-aminopyridine (4-NH2py), capable of enabling strong hydrogen-bonded intermolecular interactions.34 However, the prior solid-state examples incorporating 4-NH2py were all limited to demonstrating ion pair interactions with their halide anions, owing to their direct synthesis from I2 or IX (X = Cl, Br).34
The 3-acetaminopyridine (1) ligand was synthesised according to a literature procedure from 3-aminopyridine,35 and straightforwardly incorporated into the iodine(I) complex [I(1)2]PF6 (3) in a one-pot reaction via its respective silver(I) complex, [Ag(1)2]PF6 (2; Scheme 2). The 1H NMR spectra in CD3CN of 1–3 were as expected, showing the single alkyl peak for the acetyl group, and five peaks in the aromatic region for the four distinct aromatic groups and the NH group. The series also clearly showed the trend of moving downfield to higher frequency going from 1 to 3 (Fig. 1), with the largest coordination shift (Δδ) of 0.52 ppm observed for the aromatic hydrogen atom at the 2-position (Ha in Fig. 1) of 2 (8.78 ppm) and 3 (9.30 ppm). This trend was observed for all 1H NMR peaks except for the aromatic hydrogen atom at the 4-position (Hb in Fig. 1) of 1 (8.02 ppm) and 2 (8.02 ppm), which was unaffected by coordination to the Ag+ centre in 2.
 | ||
| Scheme 2 The synthetic procedure to generate the iodine(I) complex 3 from ligand 1via the silver(I) complex 2. | ||
The 1H–15N HMBC determined 15N NMR chemical resonances also demonstrated the same trend of coordination shifts for the pyridinic peaks of 1 (−63.7 ppm), 2 (−85.8), and 3 (−174.5 ppm), which has been previously observed for similar systems.8,32 The 15N NMR peak for 2 (−85.8) was lower than other analogous bis(substituted pyridine)silver(I) complexes reported (δN ∼110–140), but this is due to the NMR solvent being CD3CN,36,37 as opposed to the much more commonly used (and non-coordinating) solvent CD2Cl2.32 This was supported by the single crystal X-ray diffraction (SCXRD) structure of 2 (Fig. S1†), where each Ag+ centre was found to be weakly coordinated to two CH3CN molecules, with Ag⋯NCMe distances of 2.722(3) and 2.756(3) Å. This did not affect the characteristic Ag–N bond lengths of 2.183(2) and 2.189(2) Å, and a N–Ag–N angle of 174.40(8)°, which agree well with other 2-coordinate silver(I) complexes incorporating pyridine-based ligands.31
Interestingly, two solid-state structures of the same composition (polymorphs) were observed for 3, viz.3_1 and 3_2, with 3_2 containing two crystallographically independent molecules in the asymmetric unit cell (3_1 only has one molecule in the asymmetric unit). The important structural features of the two polymorphs both fall within the expected values for iodine(I) complexes, with I–N bond lengths of 2.237(5)/2.296(5) Å for 3_1, and 2.250(5)/2.251(4) Å and 2.253(4)/2.255(4) Å for the two independent molecules of 3_2, and N–I–N angles of 175.7(2)° (3_1), 177.2(2)° (3_2), and 177.8(2)° (3_2). Iodine(I) ions, and halogen(I) ions generally, do not typically demonstrate meaningful intermolecular interactions in the solid state, though some examples (I+⋯I+, I+⋯Ag+) have been reported.32,38–40 Whilst 3_2 exhibited no close contacts, with only I+⋯F–PF5 interactions of 3.779(4) Å or longer (cf. van der Waals radii of I + F = 3.45 Å), 3_1 was found to have close I+⋯F–PF5 intermolecular interactions of 3.333(9) and 3.520(6) Å from two disordered positions of the same PF6 anion. Nevertheless, the main structural difference is the arrangement of the two acetylamino groups, with 3_1 assuming a pseudo-syn conformation (40.6° between the planes of the two acetylamino groups) and both independent molecules in 3_2 assuming a pseudo-anti conformations (with 137.0° and 137.9° between the planes of the acetylamino groups).
The packing of these two polymorphs is where the differences truly emerge, with 3_1 demonstrating a 1D polymeric chain of the [I(1)2]+ cations coordinating along the axis of their N–I–N halogen bond, connected via C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N hydrogen bonding of the carbonyl oxygen atom and the NH group of adjacent cations (Fig. 2). The other pair of NH and C
O⋯H–N hydrogen bonding of the carbonyl oxygen atom and the NH group of adjacent cations (Fig. 2). The other pair of NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups per cation were found to terminate through coordination to a PF6 anion (N–H⋯F) or with no identifiable intermolecular interactions, respectively. Conversely, the packing of 3_2 was found to be constructed of 1D polymeric sheets of [I(1)2]+ cations coordinating perpendicular to the axis of their N–I–N halogen bond, and connected via pairs of C
O groups per cation were found to terminate through coordination to a PF6 anion (N–H⋯F) or with no identifiable intermolecular interactions, respectively. Conversely, the packing of 3_2 was found to be constructed of 1D polymeric sheets of [I(1)2]+ cations coordinating perpendicular to the axis of their N–I–N halogen bond, and connected via pairs of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N hydrogen bonds to each of their adjacent cations (Fig. 3).
O⋯H–N hydrogen bonds to each of their adjacent cations (Fig. 3).
These two polymorphs were reproducibly isolated from the same crystallisation solvents (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH2Cl2
1 CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN vapour diffused with pentane),‡ though at different temperatures, with crystals of 3_2 being prepared at ambient temperature, and crystals of 3_1 at 253 K. The data for the two polymorphs even had to be collected at different experimental temperatures: 200 K for 3_1, and 120 K for 3_2, which was due to the crystals of 3_1 catastrophically shattering below 200 K, even when introduced at higher temperatures and slowly cooled with ramp rates of 1 K min−1, and highlights the untapped potential of such hydrogen-bonded iodine(I) complexes to create different molecular assemblies through functional group modification and via temperature control.
MeCN vapour diffused with pentane),‡ though at different temperatures, with crystals of 3_2 being prepared at ambient temperature, and crystals of 3_1 at 253 K. The data for the two polymorphs even had to be collected at different experimental temperatures: 200 K for 3_1, and 120 K for 3_2, which was due to the crystals of 3_1 catastrophically shattering below 200 K, even when introduced at higher temperatures and slowly cooled with ramp rates of 1 K min−1, and highlights the untapped potential of such hydrogen-bonded iodine(I) complexes to create different molecular assemblies through functional group modification and via temperature control.
Upon attempted crystallisation of a CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN (7
MeCN (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution of 3 with tBuOMe over 48 hours, the unexpected reaction product [(1)CMe2(CH2I)]PF6 (4) was isolated in 65% yield (based on I+ content of 3) as a crystalline solid, with the identity of 4 definitely confirmed by SCXRD (Fig. 4). The two important features highlighted by the SCXRD analysis was the incorporation of a tBu group from the tBuOMe anti-solvent, and more interestingly, the installation of an iodine atom on a methyl group of that tBu group. As best as can be determined, no similar reactivity of iodine(I) complexes reacting with routinely used etheric solvents (commonly used as anti-solvents in the halogen(I) field) have been reported, and especially not as a major product. Similarly, precedent for the introduction of an iodine atom to an non-functionalised alkyl group is difficult to find, though the closely related field of alkyl hypoiodites reported the conversion of neopentane to neopentyl iodide in a modest yield of 34%.15 However, this reaction used in situ generated “tBuOI”, for which the structure of the actual reactive species performing the reaction is still under debate.14,15
1) solution of 3 with tBuOMe over 48 hours, the unexpected reaction product [(1)CMe2(CH2I)]PF6 (4) was isolated in 65% yield (based on I+ content of 3) as a crystalline solid, with the identity of 4 definitely confirmed by SCXRD (Fig. 4). The two important features highlighted by the SCXRD analysis was the incorporation of a tBu group from the tBuOMe anti-solvent, and more interestingly, the installation of an iodine atom on a methyl group of that tBu group. As best as can be determined, no similar reactivity of iodine(I) complexes reacting with routinely used etheric solvents (commonly used as anti-solvents in the halogen(I) field) have been reported, and especially not as a major product. Similarly, precedent for the introduction of an iodine atom to an non-functionalised alkyl group is difficult to find, though the closely related field of alkyl hypoiodites reported the conversion of neopentane to neopentyl iodide in a modest yield of 34%.15 However, this reaction used in situ generated “tBuOI”, for which the structure of the actual reactive species performing the reaction is still under debate.14,15
Whilst there is precedent for the installation of N–C bonds to aromatic amines and the activation of benzylic positions,41,42 a tBu group installed at the nitrogen position of a pyridine would still present the same synthetic challenge of introducing an iodine atom at one of the tBu methyl positions, therefore mechanistically it can be surmised that the iodine atom is introduced prior to, or concomitantly with, the introduction of the tBu substituent to a molecule of 1. As this phenomenon is unique to an iodine(I) complex incorporating ligand 1, it is also reasonable to propose that the acetylamino substituent facilitates the process in some manner.
A straightforward mechanistic explanation for 4 would be the reaction of tBuOMe with complex 3 as a source of “I+”, generating the reactive hypoiodite species IOMe and tBu+ (Fig. S7†). The tBu+ would then go on to lose a proton to become 2-methylpropene (isobutene). The alkene group of the 2-methylpropene would in turn react with a source of “I+” (either from complex 3 or the in situ generated IOMe) and a molecule of 1 (liberated from 3 to generate the initial reactive source of “I+”, [I(1)]+)43,44 would act as a nucleophile to form the new pyridinic N–C bond with the newly formed [CMe2(CH2I)]+ fragment. Whilst grounded in the literature, this proposed mechanism does not explain why this reactivity is only observed for 3 and has not been reported for other iodine(I) complexes, given that tBuOMe is a commonly used crystallisation anti-solvent, e.g., [I(quinuclidine)2]ClO4,36 or whilst tBuOMe was used as the solvent during the iodination of other substrates.45
In conclusion, the first example of a hydrogen-bonded halogen(I) complex, [bis(3-acetaminopyridine)iodine(I)]PF6 (3), was synthesised from the 3-acetaminopyridine (1) ligand via the analogous silver(I) complex 2. Complex 3 was found to possess all the expected spectroscopic and structural features of an iodine(I) complex, and two temperature-dependent crystallographic polymorphs were observed (3_1 and 3_2) that demonstrated different hydrogen-bonded connectivities between the [I(1)2]+ cations of 3. In solution, complex 3 was found to demonstrate never before observed reactivity for an iodine(I) complex, whereupon it reacted with tBuOMe to give the complex 4 in good yield, which incorporated an iodinated tBu substituent. The observation of 4 is highly novel and more extensive studies are currently underway to explore its future utilisation of 3 in organic transformations.
The author would like to acknowledge the Magnus Ehrnrooth Foundation and the University of Jyväskylä for funding this research. Dr Aaron Mailman and Prof. Kari Rissanen are thanked for useful discussions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Pancholi and P. D. Beer, Coord. Chem. Rev., 2020, 416, 213281 CrossRef CAS.
- L. Turunen, U. Warzok, R. Puttreddy, N. K. Beyeh, C. A. Schalley and K. Rissanen, Angew. Chem., Int. Ed., 2016, 55, 14033–14036 CrossRef CAS PubMed.
- A. Vanderkooy, A. K. Gupta, T. Földes, S. Lindblad, A. Orthaber, I. Pápai and M. Erdélyi, Angew. Chem., Int. Ed., 2019, 58, 9012–9016 CrossRef CAS PubMed.
- J. A. Creighton, I. Haque and J. L. Wood, Chem. Commun., 1966, 229 RSC.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed.
- J. Barluenga, J. M. González, M. A. Garcia-Martin, P. J. Campos and G. Asensio, J. Chem. Soc., Chem. Commun., 1992, 1016–1017 RSC.
- J. Ezquerra, C. Pedregal, C. Lamas, J. Barluenga, M. Pérez, M. A. García-Martín and J. M. González, J. Org. Chem., 1996, 61, 5804–5812 CrossRef CAS.
- S. Yu, J. S. Ward, K.-N. Truong and K. Rissanen, Angew. Chem., Int. Ed., 2021, 60, 20739–20743 CrossRef CAS PubMed.
- E. Kramer, S. Yu, J. S. Ward and K. Rissanen, Dalton Trans., 2021, 50, 14990–14993 RSC.
- J. L. Courtneidge, J. Lusztyk and D. Pagé, Tetrahedron Lett., 1994, 35, 1003–1006 CrossRef CAS.
- A. Yoshimura and V. V. Zhdankin, Chem. Rev., 2016, 116, 3328–3435 CrossRef CAS.
- J. Barluenga, F. González-Bobes and J. M. González, Angew. Chem., Int. Ed., 2002, 41, 2556–2558 CrossRef CAS.
- R. Kawasumi, S. Narita, K. Miyamoto, K. Tominaga, R. Takita and M. Uchiyama, Sci. Rep., 2017, 7, 17967 CrossRef PubMed.
- R. Montoro and T. Wirth, Org. Lett., 2003, 5, 4729–4731 CrossRef CAS PubMed.
- D. D. Tanner, G. C. Gidley, N. Das, J. E. Rowe and A. Potter, J. Am. Chem. Soc., 1984, 106, 5261–5267 CrossRef CAS.
- M. R. Whalon, C. H. Bushweller and W. G. Anderson, J. Org. Chem., 1984, 49, 1185–1190 CrossRef CAS.
- F. Munyemana, I. George, A. Devos, A. Colens, E. Badarau, A.-M. Frisque-Hesbain, A. Loudet, E. Differding, J.-M. Damien, J. Rémion, J. Van Uytbergen and L. Ghosez, Tetrahedron, 2016, 72, 420–430 CrossRef CAS.
- J. Li, C.-Y. Huang and C.-J. Li, Angew. Chem., Int. Ed., 2022, 61, e202112770 CAS.
- N. A. Hirscher, N. Ohri, Q. Yang, J. Zhou, J. M. Anna, E. J. Schelter and K. I. Goldberg, J. Am. Chem. Soc., 2021, 143, 19262–19267 CrossRef CAS PubMed.
- A. Paik, S. Paul, S. Bhowmik, R. Das, T. Naveen and S. Rana, Asian J. Org. Chem., 2022, 11, e202200060 CrossRef CAS.
- J. F. King, S. M. Loosmore, J. D. Lock and M. Aslam, J. Am. Chem. Soc., 1978, 100, 1637–1639 CrossRef CAS.
- J. F. King, S. M. Loosmore, M. Aslam, J. D. Lock and M. J. McGarrity, J. Am. Chem. Soc., 1982, 104, 7108–7122 CrossRef CAS.
- K. Sonogashira, J. Organomet. Chem., 2002, 653, 46–49 CrossRef CAS.
- E. Negishi, Z. Huang, G. Wang, S. Mohan, C. Wang and H. Hattori, Acc. Chem. Res., 2008, 41, 1474–1485 CrossRef CAS PubMed.
- N. V. Tzouras, I. K. Stamatopoulos, A. T. Papastavrou, A. A. Liori and G. C. Vougioukalakis, Coord. Chem. Rev., 2017, 343, 25–138 CrossRef CAS.
- R. Lakes, Nature, 1993, 361, 511–515 CrossRef.
- K. S. Katti, D. R. Katti, S. M. Pradhan and A. Bhosle, J. Mater. Res., 2005, 20, 1097–1100 CrossRef CAS.
- S. M. Zhang, F. Z. Cui, S. S. Liao, Y. Zhu and L. Han, J. Mater. Sci.: Mater. Med., 2003, 14, 641–645 CrossRef CAS PubMed.
- C. Du, F. Z. Cui, X. D. Zhu and K. de Groot, J. Biomed. Mater. Res., 1999, 44, 407–415 CrossRef CAS.
- K. Furuichi, Y. Oaki, H. Ichimiya, J. Komotori and H. Imai, Sci. Technol. Adv. Mater., 2006, 7, 219–225 CrossRef CAS.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- J. S. Ward, G. Fiorini, A. Frontera and K. Rissanen, Chem. Commun., 2020, 56, 8428–8431 RSC.
- C. Di Nicola, Effendy, F. Marchetti, C. Nervi, C. Pettinari, W. T. Robinson, A. N. Sobolev and A. H. White, Dalton Trans., 2010, 39, 908–922 RSC.
- E. Kukkonen, H. Malinen, M. Haukka and J. Konu, Cryst. Growth Des., 2019, 19, 2434–2445 CrossRef CAS.
- C. B. Aakeröy, I. Hussain, S. Forbes and J. Desper, CrystEngComm, 2007, 9, 46–54 RSC.
- J. S. Ward, A. Frontera and K. Rissanen, Dalton Trans., 2021, 50, 8297–8301 RSC.
- E. Taipale, M. Siepmann, K.-N. Truong and K. Rissanen, Chem. – Eur. J., 2021, 27, 17412–17419 CrossRef CAS.
- S. Yu, P. Kumar, J. S. Ward, A. Frontera and K. Rissanen, Chem, 2021, 7, 948–958 CAS.
- J. S. Ward, A. Frontera and K. Rissanen, Inorg. Chem., 2021, 60, 5383–5390 CrossRef CAS PubMed.
- J. S. Ward, K.-N. Truong, M. Erdélyi and K. Rissanen, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2022, DOI:10.1016/B978-0-12-823144-9.00043-1.
- W.-K. Luo, X. Shi, W. Zhou and L. Yang, Org. Lett., 2016, 18, 2036–2039 CrossRef CAS PubMed.
- J. Zhuge, Z. Jiang, W. Jiang, G. Histand and D. Lin, Org. Biomol. Chem., 2021, 19, 5121–5126 RSC.
- S. Lindblad, K. Mehmeti, A. X. Veiga, B. Nekoueishahraki, J. Gräfenstein and M. Erdélyi, J. Am. Chem. Soc., 2018, 140, 13503–13513 CrossRef CAS PubMed.
- D. von der Heiden, F. B. Németh, M. Andreasson, D. Sethio, I. Pápai and M. Erdélyi, Org. Biomol. Chem., 2021, 19, 8307–8323 RSC.
- P. Bovonsombat, W. Lorpaiboon, S. Laoboonchai, P. Sriprachaya-anunt, W. Yimkosol, N. Siriphatcharachaikul, P. Siricharoensang, T. Kangwannarakul, J. Maeda, S. Losuwanakul and M. Mahesh Abhyankar, Tetrahedron Lett., 2020, 61, 152461 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, NMR and X-ray crystallographic details. CCDC 2193903–2193906. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ce01225a |
‡ The data for 3_2 reported herein was actually collected on crystals grown by MeCN evaporation for reasons of crystal quality, however, the unit cell and packing motif of 3_2 was also identified from crystals grown from CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN (7 MeCN (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/pentane. 1)/pentane. |
| This journal is © The Royal Society of Chemistry 2022 |




