Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solid-state stability of Z′ < 1 and Z′ = 2 polymorphs of N,N,N′,N′-tetrabenzylethylenediamine: a combined experimental and theoretical study†
Zhen
Wang
a,
Xiaoxiao
Cui
a,
Antonino
Famulari
 bc,
Javier
Martí-Rujas
bc,
Javier
Martí-Rujas
 *bd,
Benson M.
Kariuki
*bd,
Benson M.
Kariuki
 *e and
Fang
Guo
*e and
Fang
Guo
 *a
*a
aCollege of Chemistry, Liaoning University, Shenyang 110036, China. E-mail: fguo@lnu.edu.cn
bDipartimento di Chimica Materiali e Ingegneria Chimica. “Giulio Natta”, Politecnico di Milano, Via L. Mancinelli 7, 20131 Milan, Italy. E-mail: javier.marti@polimi.it
cINSTM Consorzio Interuniversitario Nazionale per la Scienza e Tecnologia dei Materiali, 50121, Florence, Italy
dCenter for Nano Science and Technology@Polimi, Istituto Italiano di Tecnologia, Via Pascoli 70/3, 20133 Milano, Italy
eSchool of chemistry, Cardiff University, Main Building, Park place, Cardiff CF10 3AT, Wales, UK. E-mail: KariukiB@cardiff.ac.uk
First published on 21st October 2022
Abstract
The synthesis and structural analysis by means of single crystal X-ray diffraction (SC-XRD) and DFT calculations, of two additional new polymorphs of the flexible organic molecule N,N,N′,N′-tetrabenzylethylenediamine (L) which is used as first sphere ligand in outer sphere adducts are reported. Slow crystallization of L in the solution-state yields two polymorphs (Lα-phase and Lβ-phase) with Z′ = 0.5, while fast crystallization by rapid cooling from solution and directly from melt, allows a third, less stable polymorph with Z′ = 2 (Lγ-phase). The latter structure can be seen as a low-density metastable phase obtained by trapping L molecules after they reached high mobility by thermal treatment (i.e., high energy state). The three L polymorphs have been also studied using quantum mechanical (QM) calculations specific for the solid state by comparing the sublimation energy for each polymorph, and by comparing the experimental X-ray structures against the optimized structures from DFT, showing that Lβ-phase is the most stable and Lγ-phase is the least stable phase. The high Z′ structure can be considered as a “crystal on the way” of a more stable form. These results provide insights about crystallization mechanisms and polymorphism in organic crystals. The potential use of conformational polymorphs of flexible ligands to prepare second sphere adducts with marked polymorphism is commented.
Introduction
Polymorphism,1 the ability of a compound to exist in more than one crystalline form, is of crucial importance because polymorphs can offer a unique opportunity to study the structure–property relationships of the same compound.2,3 Studies concerning polymorphism are vital for any solid material, in particular in the pharmaceutical industry, whether single-component or multi-component (i.e., co-crystals) as two different structures of the same organic molecule can have very different physico-chemical properties.4The number of polymorphs that a substance can form is very important as it allows to gain insights in their solid-state interconversions and to understand in some cases early crystallization processes.5 While most polymorphic systems show two polymorphs, those crystallizing in three or more polymorphs are less common.6 Flexible molecules that can adopt conformations that imply low-energy conformational changes can lead to different orientations of the functional groups, and therefore give different intermolecular interactions responsible for the final crystallizing aggregates which are often metastable.
An important aspect in crystal engineering concerns the fact that some crystals contain more than one molecule in the asymmetric unit (i.e., Z′ > 1).7 The reason why Z′ > 1 occurs is still not well understood. Low symmetry and flexible molecules prone to difficult packing and highly directional hydrogen bonding could explain the occurrence of crystal structures with high Z′ values.8 Moreover, Z′ > 1 crystal structures are directly related with polymorphism and with the crystallization conditions that allow to explore as many as possible molecular conformations with different lattice energies before nucleating and crystallizing in a solid form.9–11 Therefore, studies providing additional examples of structures with more than one molecule in the asymmetric unit are indeed needed for its better understanding.
Derivative ligands of N,N,N′,N′-tetrabenzylethylenediamine (L) have been used to great effect for the synthesis and solid-state reactivity of metal organic materials by using second sphere interactions.12 However, to the best of our knowledge, polymorphic studies of bidentate ligand L have not been reported despite its use in the preparation of metal organic materials by means of coordination chemistry. Interestingly, a flexible ligand such as L with strong polymorphic behaviour can induce polymorphism in coordination complexes (i.e., metal organic frameworks (MOFs)11 and other metal–organic complexes),13 due to the inherent structural versatility.
The aim of the current work is to study the polymorphic behaviour of the benzylethylenediamine derivative L using various crystallization methods and the solvent effect. Because the ligand has several torsional angles among the pendant benzyl groups and the flexible central alkyl chain, it can yield different polymorphs. The rationale for choosing this molecule is because derivatives of L ligands, either by changing the length12,14 and the nature15 of the central backbone or by functionalizing the external aromatic rings16 are being used to synthesize metal organic materials applying second sphere interactions.13 It has been observed that few outer sphere structures have been obtained showing polymorphic behavior.14,15 Furthermore, our interest in the polymorphism of L is also because it can give us insights on the solid-state reactivity of the second sphere adducts (i.e., dehydrochlorination reactions) which may depend on the intrinsic polymorphic behavior of the organic part of the metal–organic materials.
Here, we report on the structural properties of three polymorphs of the N,N,N′,N′-tetrabenzylethylenediamine ligand (L) (Scheme 1) which has been recently used in the synthesis of outer sphere adducts.13,14 The different polymorphs are obtained by varying the crystallization conditions. Two of the polymorphs have half molecule of L in the asymmetric unit (Z′) and are obtained via slow crystallization methods upon evaporation. While the third one, is obtained by fast crystallization from solution or melt and contains two independent molecules of L in the asymmetric unit (Z′ = 2). The thermodynamic stability among the three polymorphs showed that the least stable corresponds to a metastable phase with Z′ = 2 (i.e., the less dense structure). This has been demonstrated by experimental results and corroborated using density functional theory (DFT) calculations specific for solid-state systems. Interestingly the metastable phase can be obtained upon heating followed by cooling of one of the polymorphs with Z′ = 0.5 (stable phase). This could be considered as a high energy phase (mesophase) of the crystal structure before reaching melting and fits with the theory that Z′ > 1 is just one of the many options that organic molecules (i.e., L) can take during the crystallization process (or just before it melts). Our point of view on whether a ligand with high tendency to form polymorphs might also induce polymorphism in second sphere adducts is given.
Results and discussion
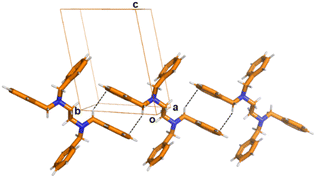
The list of ethylenediamine derivative ligands that are flexible and potential candidates to form conformers is vast. For instance N,N,N′,N′-tetrabenzylpropydiamine, N,N,N′,N′-tetrabenzylbutyldiamine, N,N,N′,N′-tetra-p-methoxybenzyl-ethylenediamine, (R,R)-N,N′-dibenzyl-1,2-diaminocyclohexane, N,N,N′,N′-p-methyl-tetrabenzyl-ethylenediamine or N,N,N′,N′-p-trifluoromethyl-tetrabenzyl-ethylenediamine, are bidentate ligands with potential formation of polymorphs. For the focus of this work the ligand N,N,N′,N′-tetrabenzyl-ethylenediamine (L) was selected because it has the shortest central backbone chain –N–CH2–CH2–N– that forms second sphere adducts undergoing dehydrochlorination reactions.14Ligand L was synthesized according to literature.17 The obtained product was recrystallized using various crystallization conditions giving three polymorphs which are described hereafter. Recrystallization by evaporation of the as synthesized L was carried out at ambient conditions using a variety of different solvents such as methanol, ethanol, acetone, THF and DMF. In all the cases, block-shape colorless single crystals suitable for single crystal X-ray (SC-XRD) analysis were obtained (Scheme 1). Crystallographic analysis18 revealed that the unit cell and space group are the same as those already reported. Herein, the reported phase is labelled as (Lα-phase).19Lα-phase crystallizes in the low symmetry triclinic system in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The asymmetric unit contains half L molecule. As shown in Fig. 1, C–H⋯π interactions and π⋯π stacking are the main driving forces for the stabilization of the structures. Interestingly, powder X-ray diffraction (powder XRD) analysis of the bulk material clearly showed that additional peaks to Lα-phase were observed, thus indicating that a new extra phase was obtained (Fig. S1†). Therefore, we named the new phase Lβ-phase. In most cases from the crystallization experiments, the yield of Lα-phase was higher than that of Lβ-phase.
space group. The asymmetric unit contains half L molecule. As shown in Fig. 1, C–H⋯π interactions and π⋯π stacking are the main driving forces for the stabilization of the structures. Interestingly, powder X-ray diffraction (powder XRD) analysis of the bulk material clearly showed that additional peaks to Lα-phase were observed, thus indicating that a new extra phase was obtained (Fig. S1†). Therefore, we named the new phase Lβ-phase. In most cases from the crystallization experiments, the yield of Lα-phase was higher than that of Lβ-phase.
 | ||
| Fig. 1 Crystal structure of the Lα-phase. Short C–H⋯π contacts are depicted by black dashed lines. Colour code: carbon, orange; nitrogen, blue and hydrogen, white. | ||
Crystal structure description of Lα-phase
As seen in Fig. 1, in Lα-phase the two methylene groups belonging to the phenyl rings in one molecule form C–H⋯π interactions with the neighbouring molecules, forming a chain along the b-axis (dC–H⋯π = 3.668(4) Å, angle ∠dC–H⋯π = 139°) (Fig. 1). No other significant weak interactions are observed. As mentioned,14 the angles N1–C2–C3 (113°) and N1–C9–C10 (114°) differ significantly from the regular tetrahedral value of (109.5°) due to the steric hindrance brought by the vicinity of benzene rings.Crystal structure description of Lβ-phase
Even though all the single crystals look similar (i.e., colorless blocks), a careful screening using optical microscopy and SC-XRD allowed the identification of a crystal with a different unit cell (Table 1), thus corroborating the powder XRD results. X-ray crystallography revealed that the new crystal (i.e., Lβ-phase) also crystallizes in the triclinic system (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) and contains half molecule in the asymmetric unit.
) and contains half molecule in the asymmetric unit.
| L α-phase 14 | L β-phase | L γ-phase | |
|---|---|---|---|
| Temperature | 293 K | 296 K | 293 K |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
C2/c |
| a (Å) | 5.872(1) | 6.356(4) | 39.090(1) |
| b (Å) | 10.202(3) | 9.042(6) | 12.916(1) |
| c (Å) | 10.629(3) | 11.117(7) | 20.068(1) |
| α (°) | 97.62(1) | 76.99(1) | 90 |
| β (°) | 101.62(1) | 86.68(1) | 93.66(1) |
| γ (°) | 93.95(1) | 79.28(1) | 90 |
| V (Å3) | 615.2(3) | 611.5(7) | 10111.8(7) |
| D x (g cm−3) | 1.135 | 1.142 | 1.105 |
| μ (mm−1) | 0.07 | 0.07 | 0.49 |
| F (000) | 226 | 3616 | |
| R int | 0.03 | 0.02 | 0.03 |
| Total reflections | 3224 | 2690 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 079 079 |
| Unique reflections | 2166 | 2033 | 9184 |
| Observed refs. (I > 2σ(I)) | 1283 | 1418 | 6046 |
| Refined parameters | 146 | 145 | 578 |
| R f/wRf | 0.05/0.18 | 0.05/0.13 | 0.07/0.20 |
| All data Rf/wRf | 0.10/0.15 | 0.09/0.23 | |
| GoF | 1.06 | 1.08 | 1.05 |
In the crystal structure of the Lβ-phase, one methylene group takes part in the C–H⋯π electrostatic interaction's network (dC–H⋯π = 3.630(3) Å, angle ∠dC–H⋯π = 138°) giving rise to a chain expanding approximately along the crystallographic b-axis (Fig. 2). In Lβ-phase the deviation from the standard 109.5° is less pronounced for the N1–C1–C2 (112°) and N1–C8–C9 (114°). The overall structures of Lα-phase and Lβ-phase are nearly identical, which might be an important aspect explaining their co-existence in the same crystallization batch (i.e., concomitant polymorphs). The simulated powder XRD of Lα-phase and Lβ-phase are markedly different (see ESI†).
 | ||
| Fig. 2 Crystal structure of Lβ-phase. Short C–H⋯π contacts are depicted by black dashed lines. Colour code as in Fig. 1. | ||
On the view of the polymorphic behaviour of L, we thought to explore different crystallization methods. According to literature reports,7d crystal structures with Z′ > 1 can be obtained by rapid crystallization as most Z′ > 1 structures are considered kinetic (i.e., metastable) products. Therefore, we explored crystallization of L by fast cooling methods prone to give kinetic products. Rapid crystallization from a supersaturated methanol solution of L at high temperature (i.e., nearly methanol boiling point (65 °C)) yielded a new phase (Lγ-phase) with a completely different habit (i.e., long needles) as shown in Scheme 1. Despite the fast crystallization, the single crystals obtained were of good quality for SC-XRD analysis. Interestingly, when the solution containing the crystallized long needles were left standing at room temperature, the crystals dissolved within minutes, thus indicating that they belong to a metastable phase. Then, we turned out to explore if the Lγ-phase can be obtained from the melt which has been also claimed to have high chances to give structures with Z′ > 1.20 Melting L followed by rapid cooling (quenching) at room temperature gave long colourless needles suitable for SC-XRD. X-ray crystallography showed that the new phase corresponds to Lγ-phase with remarkably different unit cell parameters compared to Lα-phase and Lβ-phase (Table 1). Crucially, in the bulk sample there is no presence of Lα-phase and Lβ-phase, and therefore crystallization from the melt allows a selective way to obtain this polymorph.
Crystal structure description of Lγ-phase
The Lγ-phase crystallizes in the monoclinic system in the C2/c space group. Notably, there are two independent molecules in the asymmetric unit with different conformations, herein labelled as conformers LA and LB, respectively (Fig. 3). That means that Z′ = 2. The unit cell of Lγ-phase is quite different compared to those of Lα-phase and Lβ-phase. We note that the unit cell volume of Lγ-phase is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112 Å3 while that of Lα-phase and Lβ-phase are 615 Å3 and 611 Å3 respectively (Table 1). This is a remarkable change in unit cell volume showing that interactions in the high temperature phase, prevails over packing in the thermodynamic structure.
112 Å3 while that of Lα-phase and Lβ-phase are 615 Å3 and 611 Å3 respectively (Table 1). This is a remarkable change in unit cell volume showing that interactions in the high temperature phase, prevails over packing in the thermodynamic structure.
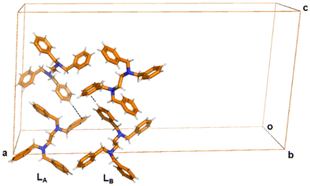 | ||
| Fig. 3 Crystal structure of Lγ-phase. Short C–H⋯π contacts are depicted by black dashed lines. The two independent molecules in the asymmetric unit are labelled LA and LB. The intermolecular interactions are clearly different from the Lα-phase and Lβ-phase. Colour code as in Fig. 1. | ||
The three-dimensional arrangement involves a different set of C–H⋯π interactions between LA and LB, independent molecules, by means of C21–H21⋯π interactions (dC–H⋯π 3.751(6) Å, angle ∠dC–H⋯π = 176°). We note that the C–H⋯π interaction is different from the C–H⋯π interaction mode in Lα-phase and Lβ-phase, in which the hydrogen bonding donor comes from the benzene ring instead of methylene group (Fig. 3).
A comparison among three phases is also made in terms of the density and overall packing efficiency. The density of phase γ (1.105 g cm−3) is lower than those of phases α (1.135 g cm−3) and β (1.142 g cm−3). The packing efficiencies are 70.5% for α, 71.1% for β and 69.6% for γ, respectively. The greater density of β correlates with its greater packing efficiency when compared to that of α and γ.
The IR spectra of three polymorphs of L were quite similar, as seen in Fig. S2.† The most distinguishable differences in the IR spectra of the Lα-phase, Lβ-phase and Lγ-phase are in the C–H bending of benzene rings (782 cm−1 for Lγ-phase, 780 cm−1 for phase Lα-phase/Lβ-phase), indicating the different molecular environments of CH and CH2 groups could be involved in the formation of different short contacts. This is consistent with the crystal structures studies of polymorphs Lα-phase, Lβ-phase and Lγ-phase. Table 1 shows the crystallographic details of the three L polymorphs described herein.
Analysis of isolated L molecules shows that the conformations in Lα-phase and Lβ-phase are very similar (see Fig. 4a), while the two conformations observed in Lγ-phase (LA and LB) are significantly different from the Lα-phase and Lβ-phase (Fig. 4b). Moreover, the conformational energy of Lγ-phase is higher than that of Lα-phase and Lβ-phase, so that L molecules need to be trapped into higher conformational energy levels to arrange into the Lγ-phase crystalline structure (i.e., in this case thermal treatment).
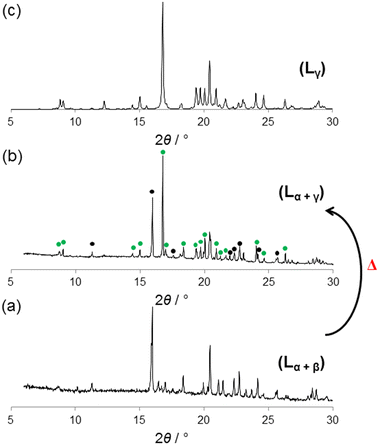
Thermodynamic stabilities of Lα-phase, Lβ-phase and Lγ-phase
To gain insights on the relative thermodynamic stabilities of the three L polymorphs, powder XRD experiments upon heating were carried out. The starting material is a mixture of Lα-phase and Lβ-phase due to their concomitant crystallization (see Fig. 5a).21 The sample was heated22 from room temperature to 90 °C and annealed for 1 h and measured by in situ powder XRD. As shown in Fig. S3,† the phase transition from Lα-phase to the Lγ-phase did not occur until ca. 80 °C. At 80 °C, with the Lβ-phase disappearing, and the intensity of Lγ-phase, particularly the peak at 2θ = 16°, is significant (Fig. 5b). Upon annealing at 90 °C for 6 h the Lγ-phase is the predominant one although the transformation is not completed.23 However, there is no doubt that upon heating the phase that forms is the Lγ-phase (Fig. 5).DSC was also used to monitor the thermal behavior of L. For both a freshly crystallized sample containing a mixture of phases α and β (Fig. S4†) and phase γ (Fig. S5†) there is no phase transition below 90 °C, even at a heating rate of 1 °C min−1. This is apparently inconsistent with the powder XRD data but it should be noted that the two experiments followed different protocols. In Fig. S4,† the small exothermic peak at 92.4 °C is associated with the transformation of α and β to γ (with β possibly transforming through α as an intermediate). The larger endothermic peak at 94.7 °C is due to the melting of the γ phase. This was confirmed by heating a fresh sample to just below 94 °C which was then subjected to an ex situ powder XRD experiment. The powder XRD pattern of the product (Fig. S6a†), shows that the solid is the γ phase due to the good match among both diffractograms (Fig. S6b†).
DFT structural analysis
To gain insights on the relative stabilities among the three polymorphs described, we have carried out DFT calculations specific for the solid state. Complementary information in the field of solid-state chemistry can be obtained by theoretical methods and in particular QM calculations specific for solid phases. Herein, DFT approaches have been used. The GGA–PBE Perdew–Burke–Ernzerhof (PBE)24 exchange–correlation functional has employed both for gas and solid phase (i.e., under periodical conditions) calculations. For the sake of consistency and to maintain reasonable computational costs, all calculations were carried out using the DMOL3 software.25In all the calculations, we used the experimental X-ray determined unit cells while relaxing atomic coordinates of all atoms. The same approach has been used for similar systems26 and for other organic crystalline phases.27 A numerical double-ζ quality basis set [including polarization functions on all atoms, i.e., double-ζ numerical with polarization (DNP)], roughly comparable with the usual 6-31G** Gaussian basis, was adopted. The inclusion of explicit van der Waals terms in the calculations has been chosen because of their importance when describing inter-particle interactions.28
DFT results show that Lγ-phase is the less stable polymorph. In fact, the estimated sublimation energy (E*) (i.e., the energy required to extract a single molecule from the corresponding crystal structure) for Lγ-phase is about 10 kcal mol−1 lower with respect to that of Lα-phase and Lβ-phase. This agrees with the experimental data reported and, with the fast crystallisation conditions required for the formation of the Lγ-phase (i.e., the trapping of a metastable phase). The DFT calculations also show that Lβ-phase is only ca. 1.5 kcal mol−1 more stable with respect to Lα-phase. We note that the short contacts are closer in the Lβ-phase than in the Lγ-phase, suggesting a higher stability.
Comparison among experimental and DFT optimized structures (i.e., alpha, beta and gamma structures)
Because the changes among the various described phases are small, we decided to have a closer look at the optimized Lα-phase, Lβ-phase and Lγ-phase structures and compare them with the X-ray experimental structures to see any significant changes that might help to understand the observed polymorphism, in particular their relative stabilities.Importantly, in the Lα-phase the C–H⋯π interaction between a methyl group from the benzene ring that yields the chain along the b-axis shown in Fig. 1 is still maintained with shorter distances with respect to the room temperature data (experimental). The optimized C–H⋯π distance is dC–H⋯π = 3.650 Å and angle ∠dC–H⋯π = 139°. As expected for a low temperature data, several other weak interactions are present such as the one including the second methylene group belonging to the phenyl rings is also observed with the following geometries dC–H⋯π = 3.617 Å and angle ∠dC–H⋯π = 151°. Additionally, the two central methylene groups in the ethylenic backbone interact with neighbouring aromatic rings via repulsive interactions by means of C–H⋯H contacts (dC–H⋯H = 3.365 Å, angle ∠dC–H⋯H = 154° and dC–H⋯H = 3.395 Å, angle ∠dC–H⋯H = 160°). For this polymorph, no major changes are observed in the optimized structure compared to the experimental data.
The Lβ-phase polymorph is very similar to the alpha phase (i.e., differing in only 1.5 kcal mol−1 in the sublimation energy calculated in both phases) (Fig. 6), and the main interaction used to describe the room temperature structure between the methylene group from the aromatic ring and adjacent benzene ring shown in Fig. 2 is dC–H⋯π = 3.808 Å with angle ∠dC–H⋯π = 142°. The Lβ-phase also shows a second CH2 from the benzene ring interacting with an adjacent aromatic ring. This second contact is stronger with distance dC–H⋯π = 3.543 Å and angle ∠dC–H⋯π = 136°. Interestingly, in this case the beta polymorph does not present the repulsive C–H⋯H contacts involving the ethylenic backbone, which corroborates the slightly higher stability seen in this phase with respect to the alpha phase. No big structural differences are observed between the experimental and optimized structure in the Lβ-phase.
 | ||
| Fig. 6 Plot showing the relative sublimation energies (E*) calculated using DFT for Lα-phase, Lβ-phase and Lγ-phase. The lower E* denotes that the gamma phase is the less stable polymorph. | ||
In the Lγ-phase, the short interactions are described for the independent LA and LB molecules in the asymmetric unit. In the optimized structure the main interaction, which is among two aromatic rings via C–H⋯π used for the description of the structure at room temperature (Fig. 3), shows a shorter C–H(LB)⋯C(LA) distance dC–H⋯π = 3.692 Å, ∠dC–H⋯π = 175° which is expected for the low temperature (DFT) data. In the independent LA molecule, only one CH2 from a benzene group shows attractive C–H⋯π contacts (dC–H⋯π = 3.690 Å, ∠dC–H⋯π = 140°) with the other three methylene groups left without interactions. The two central ethylenic CH2 groups interact via repulsive C–H⋯H contacts with adjacent L molecules (dC–H⋯H 3.007 Å, ∠dC–H⋯H 119° and dC–H⋯H 3.087 Å, ∠dC–H⋯H 121°). In the other independent LB molecule, the four CH2 groups belonging to the aromatic ring show different interactions. One of the CH2 group is interacting via attractive C–H⋯π contacts (dC–H⋯π = 3.723 Å, ∠dC–H⋯π = 140°), stabilizing the structure. However, two CH2 groups interact via repulsive interactions (dC–H⋯H 3.125 Å, ∠dC–H⋯H 129° and dC–H⋯H 3.112 Å, ∠dC–H⋯H 124°) and one methylene group is not showing any short contact. The two CH2 groups in the ethylenic backbone do not show interactions. Also in this case, no major structural differences are observed in the experimental X-ray data compared to the optimized DFT structures in the Lγ-phase.
According to the above-described weak interactions, the more stable structure among the very similar alpha and beta phases, is the beta phase, the one showing less repulsive interactions. This stability shows a good correlation with the idea that Z′ with lower values are regarded thermodynamic structures. In fact, in the alpha and beta phases Z′ = 0.5. The gamma phase with Z′ = 2 is the less stable polymorph as confirmed by the presence of more repulsive interactions compared to Lα-phase and Lβ-phase. This is also explained by the presence of the two L conformers in the asymmetric unit as more intermolecular interactions can be established. Thus, the DFT optimized structures show a good agreement with the experimental data and with the computed sublimation energies used to determine the relative thermodynamic stabilities among polymorphs. The following polymorphic stability order Lβ-phase > Lα-phase > Lγ-phase is therefore confirmed by the DFT calculations (Fig. 6).
Kinetic trapping of L with Z′ = 2 by fast crystallization: a snapshot in the crystallization process towards Z′ < 2
Crystal structures with Z′ > 1 have been reported to be considered as “crystals on the way”, as “fossil relics of the fastest growing crystal nucleus” or as different “snapshot pictures at different stages of crystallization”. Desiraju and co-workers29 stated that Z′ > 1 can be just one of the many options that organic molecules can take during the crystallization process. Generally, packing problems will tend to lead towards structures having Z′ > 1 due to awkward molecular geometries and flexible conformations. From our experimental data, upon heating and fast cooling, the Lα-phase and Lβ-phase transforms into the Lγ-phase which is metastable (i.e., lower E* from DFT calculations). The extra thermal energy given to the solid implies that due to higher atomic mobility the conformation in L changes (i.e., ligand L contains eleven torsion angles) giving the arrangement of two L conformers (LA and LB) in the crystalline state which can be trapped as a metastable phase. If the solid melts or becomes a mesophasic state, upon fast cooling it will crystallize as the Lγ-phase. Therefore, the Lγ-phase can be considered as the metastable structure. In our opinion, the Lγ-phase corresponds to a snapshot that captures a picture of a crystallization event where a high energy phase (i.e., metastable phase) with Z′ = 2 is trapped in the crystalline state by means of fast crystallization events that do not allow L to reach the most thermodynamically stable conformation. The combination of flexible (ethylenic backbone) and rigid groups (benzene rings) in ligand L is contributing to the observed polymorphism described in this work.L polymorphism and second sphere coordination adducts
From the combined X-ray and DFT work reported herein, we have observed that the difference in the sublimation energies (E*) among the three polymorphs is within 10 kcal mol−1 (Fig. 6). It is important to notice that such energies are the typical energies associated with intramolecular torsions.30 Although much weaker, van der Waals and dispersive interactions present in the three polymorphs reported are in the order of 0.5–1.0 kcal mol−1. The computed difference in E* among Lα-phase, and Lβ-phase is quite small (1.5 kcal mol−1) but in the order of magnitude mentioned above. Higher energy is needed to form the Lγ-phase, which is the polymorph formed because more significant changes in the torsional angles are observed, that gives two L conformers in the asymmetric unit. Thus, the intrinsic flexibility of L is giving different conformers (LA and LB) in the form of polymorphs. In particular, the less stable phase can be obtained selectively as a kinetic form from the melt by fast crystallization.The polymorphic behaviour of L studied in this work is relevant because gives insights about structural variability that can be exploited also in hybrid metal organic materials self-assembled using non-covalent second sphere interactions.31 Since organic ligands, in this case L, are used as first sphere ligands in combination with transition metals, the polymorphic behaviour of L can thus induce polymorphism (i.e., can be translated) to the second sphere adduct formed by the combination of metal anions and protonated ligands. In a second sphere adduct, the organic component can drive the formation of an outer sphere polymorph rather than the metal ion. In this regard, it was demonstrated that in the solid-state by mechanochemical means the second sphere adduct [LH]+·[FeCl4]−, (where L is N,N,N′,N′-tetrabenzyl-trans-1,2-diaminocyclohexane) formed selectively one polymorph that crystallized in the polar space group Pna21 displaying NLO properties.
Although in this work only one ligand (L) has been studied, our aim is to monitor the polymorphic behaviour of L derivatives, to explore if a marked polymorphic behaviour of a given ligand (i.e., L derivative) can form a high number of polymorphs with high Z′ when such ligand is employed as first sphere ligand in outer sphere adducts. Despite the long history of second sphere interactions, polymorphism on hybrid metal–organic materials has not been explored much (i.e., compared to polymorphism of organic molecules and pharmaceuticals). Therefore, the reported results not only are of interest for the L itself, but also because other polymorphs can be formed using L and metals in outer sphere adducts.
Conclusions
In conclusion, we have reported structural aspects of three polymorphs of the flexible bidentate ligand L (Lα-phase, Lβ-phase and Lγ-phase) that differ in the geometry and number of molecules in the asymmetric unit. Polymorphs Lβ-phase and Lγ-phase are reported for the first time. Slow crystallization yields two polymorphs with Z′ = 0.5, while fast crystallization directly from melt allows a third polymorph with Z′ = 2. Our interpretation is that the polymorph with Z′ = 2 is a metastable structure that can form only by fast crystallisation and is observed experimentally by powder XRD upon heating, and by fast cooling the more stable structures with Z′ = 0.5 are formed. Theoretical QM calculations specific for solid-state systems have demonstrated that the structure with Z′ = 2 is the less stable among the three polymorphs, thus corroborating the theory that the polymorph with Z′ = 2 is a metastable structure which is kinetically trapped by fast crystallization events. As coined by Steed32 that high Z′ structure are “crystals on the way” of a more stable form, our results reported herein just confirm that statement. It is our opinion that a ligand which forms various polymers might be used to form also polymorphic second sphere adducts by using non-covalent electrostatic interactions. Research in this direction is being carried in our labs to explore crystallization conditions that can trap crystalline structures with high Z′ numbers in L derivatives and in second coordination spheres self-assembled using L derivatives and transition metal ions.Experimental
All chemicals were obtained from commercial sources and used without further purification. Powder XRD were recorded at room temperature on a D8 Bruker diffractometer, with Cu-Kα1 radiation (λ = 1.54056 Å) and a step size of 0.01°, step time of 0.2 s. High temperature powder XRD experiments were performed in reflection (Bragg–Brentano) geometry using a Bruker D8 powder diffractometer with CuKα1 radiation. An Anton Paar HTK 1200 furnace was used in the variable-temperature study. The temperatures are accurate to ±2 °C. Samples (about 100 mg) were placed in a sample holder and smoothed with a glass slide. Data were collected in the range 5° < 2θ < 30° with a step size of 0.02°. Differential scanning calorimetry (DSC) was performed using a TA Instrument Q100 differential scanning calorimeter. Small amounts (5–10 mg) of sample were weighed and sealed in DSC pans and placed in the sample chamber of the calorimeter. Heating/cooling rates were tuned during the DSC experiment, 20 °C min−1 in the range of 20–80 °C and 110–20 °C, and 1 °C min−1 in the range of 80 to 110 °C.Single crystal X-ray diffraction for Lβ-phase was carried out using a Bruker D8 QUEST X-ray single crystal diffractometer. An Agilent SuperNova Dual Atlas diffractometer was used for phase Lγ-phase. Crystal structures were solved and refined using SHELXS and SHELXL.33,34 Non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were inserted in idealized positions, and a riding model was used with Uiso set at 1.2 times the value of Ueq for the atom to which they are bonded. Crystallographic data for phases Lβ-phase and Lγ-phase have been deposited with the Cambridge Crystallographic Data Center (CCDC) under reference numbers 2109973 and 2108276, respectively.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was supported by National Science Foundation of China (No. 21571090), and Liaoning BaiQianWan Talents Program, and Project of Education Department of Liaoning Province of China (LJKZ0102). A. F. acknowledges MIUR for FFARB “Fondo finanziamento delle attività base di ricerca” and CINECA for computational resources.References
- (a) J. Bernstein, Polymorphism in Molecular Crystals, OUP, Oxford, 2002 Search PubMed; (b) S. L. Price, Acc. Chem. Res., 2009, 42, 117 CrossRef CAS PubMed; (c) J.-P. Brog, C.-L. Chanez, A. Crochet and K. M. Fromm, RSC Adv., 2013, 3, 16905–16931 RSC; (d) A. J. Cruz-Cabeza and J. Bernstein, Chem. Rev., 2014, 114, 2170 CrossRef CAS PubMed; (e) A. J. Cruz-Cabeza, S. M. Reutzel-Edens and J. Bernstein, Chem. Soc. Rev., 2015, 44, 8619 RSC; (f) A. J. Cruz-Cabeza, N. Feeder and R. J. Davey, Commun. Chem., 2020, 3, 142–146 CrossRef CAS.
- (a) B. M. Kariuki, C. L. Bauer, K. D. M. Harris and S. J. Teat, Angew. Chem., Int. Ed., 2000, 39, 4485–4487 CrossRef CAS; (b) M. Xu and K. D. M. Harris, J. Phys. Chem. B, 2007, 111, 8705–8707 CrossRef CAS PubMed.
- (a) P. Sanphui, B. Sarma and A. Nangia, J. Pharm. Sci., 2011, 6, 2287–2299 CrossRef PubMed; (b) Y. Zhou, F. Guo, C. E. Hughes, D. L. Browne, T. R. Paskett and K. D. M. Harris, Cryst. Growth Des., 2015, 15, 2901–2907 CrossRef CAS.
- (a) S. Aitipamuka, P. S. Chow and R. B. H. Tan, CrystEngComm, 2014, 16, 3451–3465 RSC; (b) K. Xu, X. Xiong, L. Guo, L. Wang, S. Li, P. Tang, J. Yan, S. Wu and H. Li, J. Pharm. Sci., 2015, 104, 4123 CrossRef CAS PubMed; (c) N. K. Duggirala, M. L. Perry, O. Almarsson and M. J. Zarowotko, Chem. Commun., 2016, 52, 640 RSC; (d) S. A. Ross, D. A. Lamprou and D. Douromis, Chem. Commun., 2016, 52, 8772 RSC; (e) H. Kulla, S. Greiser, S. Benemann, K. Rademann and F. Emmerling, Cryst. Growth Des., 2017, 17, 1190 CrossRef CAS; (f) R. Kaur, S. Cherukuvada, P. B. Managutti and T. N. G. Row, CrystEngComm, 2016, 18, 3191–3203 RSC; (g) P. Bora, B. Saikia and B. Sarma, Chem. – Eur. J., 2020, 26, 699–710 CrossRef CAS PubMed; (h) S. Aitipamula and L. P. Shan, CrystEngComm, 2022, 24, 560–570 RSC.
- (a) C. E. Hughes and K. D. M. Harris, J. Phys. Chem. A, 2008, 112, 6808–6810 CrossRef CAS PubMed; (b) C. E. Hughes and K. D. M. Harris, Chem. Commun., 2010, 46, 4982–4984 RSC; (c) P. C. Vioglio, G. Mollica, M. Juramy, C. E. Hughes, P. A. Williamos, F. Ziarelli, S. Viel, P. Thurreau and K. D. M. Harris, Angew. Chem., Int. Ed., 2018, 57, 6619–6623 CrossRef PubMed.
- (a) L. Yu, G. A. Stephenson, C. A. Mitchell, C. A. Bunnell, C. A. Snorek, J. J. Bowyer, T. B. Borchardt, J. G. Stowell and S. R. Byrn, J. Am. Chem. Soc., 2000, 122, 585 CrossRef CAS; (b) C. A. Mitchell, L. Yu and M. D. Ward, J. Am. Chem. Soc., 2001, 123, 10830 CrossRef CAS PubMed; (c) S. Chen, I. Guzei and L. Yu, J. Am. Chem. Soc., 2005, 127, 9881 CrossRef CAS PubMed; (d) P. A. Williams, C. E. Hughes, G. K. Lim, B. M. Kariuki and K. D. M. Harris, Cryst. Growth Des., 2012, 12, 3104–3113 CrossRef CAS; (e) S. Ahn, F. Guo, B. M. Kariuki and K. D. M. Harris, J. Am. Chem. Soc., 2006, 128, 8441–8452 CrossRef CAS PubMed.
- (a) N. J. Babu and A. Nangia, Cryst. Growth Des., 2006, 6, 1995–1999 CrossRef CAS; (b) M. Rafilovich and J. Bernstein, J. Am. Chem. Soc., 2006, 128, 12185–12191 CrossRef CAS PubMed; (c) S. Mahapatra, T. S. Thakur, S. Joseph, S. Varughese and G. R. Desiraju, Cryst. Growth Des., 2010, 10, 3191–3202 CrossRef CAS; (d) E. M. Brás, M. S. C. Henrique, J. A. Paixao and R. Fausto, Cryst. Growth Des., 2018, 18, 4167–4173 CrossRef; (e) J. B. Nanubolu, Cryst. Growth Des., 2021, 21, 133–148 CrossRef CAS; (f) N. Caimac, E. Melnic, D. Chisca and M. S. Fonari, CrystEngComm, 2021, 23, 3099–3108 RSC.
- J. W. Steed, CrystEngComm, 2003, 5, 169–179 RSC.
- K. M. Steed and J. W. Steed, Chem. Rev., 2015, 115, 2895–2933 CrossRef CAS PubMed.
- G. R. Desiraju, CrystEngComm, 2007, 9, 91–92 RSC.
- C. S. Hawes, N. F. Chilton, B. Moubaraki, G. P. Knowles, A. L. Chaffee, K. S. Murray, S. R. Batten and D. R. Turner, Dalton Trans., 2015, 44, 17494–17507 RSC.
- F. Guo, X. Wang, H.-Y. Guan, H.-B. Yu, L. Li, S.-S. Chen, A. Famulari and J. Martí-Rujas, Cryst. Growth Des., 2015, 15, 2842–2852 CrossRef CAS.
- (a) F. Guo and J. Martí-Rujas, Dalton Trans., 2016, 45, 13648–13662 RSC; (b) F. Guo and J. Martí-Rujas, Dalton Trans., 2021, 51, 11665–11680 Search PubMed.
- H. Y. Guan, Z. Wang, A. Famulari, X. Wang, F. Guo and J. Martí-Rujas, Inorg. Chem., 2014, 53, 7438 CrossRef CAS PubMed.
- H. B. Yu, H. T. Li, P. Zhang, A. Famulari, F. Guo, I. Bargigia and J. Martí-Rujas, CrystEngComm, 2016, 18, 2408–2412 RSC.
- F. Guo, Z. Wang, J.-J. Zhang, A. Famulari, H.-T. Li and J. Martí-Rujas, Dalton Trans., 2017, 46, 9466–9471 RSC.
- Z. Pan, M. Zhang, D. Yuan and P. Ma, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2005, 61, o185–o186 CrossRef CAS.
- SC-XRD structure analysis was carried out at room temperature.
- FAYHAP is the CCDC code for the Lα-phase described in ref. 14.
- B. Sarma, S. Roy and A. Nangia, Chem. Commun., 2006, 4918–4920 RSC.
- J. Bernstein, R. J. Davey and J.-O. Henck, Angew. Chem., Int. Ed., 1999, 38, 3440–3461 CrossRef PubMed.
- Heating conditions: at 50 °C, 60 °C, 70 °C, 80 °C, 85 °C and 90 °C respectively.
- Complete transformation has been obtained upon melting in DSC. See ESI.†.
- (a) J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed; (b) J. P. Perdew, K. Burke and M. E. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396–1396 CrossRef CAS.
- B. J. Delley, Chem. Phys., 2000, 113, 7756 CAS.
- (a) H. Yu, L. Li, J. Gao, J. Tong, W. Zheng, M. Cametti, A. Famulari, S. V. Meille, F. Guo and J. Martí-Rujas, Dalton Trans., 2015, 44, 15960–15965 RSC; (b) F. Guo, M.-Q. Zhang, A. Famulari and J. Martí-Rujas, CrystEngComm, 2013, 15, 6237–6243 RSC.
- (a) A. Famulari, G. Raos, A. Baggioli, M. Casalegno, R. Po and S. V. Meille, J. Phys. Chem. B, 2012, 116, 14504–14509 CrossRef CAS PubMed; (b) T. Nicolini, A. Famulari, T. Gatti, J. Martí-Rujas, F. V. Monteleone, E. V. Canesi, C. Botta, E. Parisini, S. V. Meille and C. Bertarelli, J. Phys. Chem. Lett., 2014, 5, 2171–2176 CrossRef CAS PubMed.
- (a) S. Grimme, J. Chem. Phys., 2006, 124, 34108–34134 CrossRef PubMed; (b) A. Baggioli, S. Meille, G. Raos, R. Po, M. Brinkmann and A. Famulari, Int. J. Quantum Chem., 2013, 113, 2154–2162 CrossRef CAS; (c) A. Baggioli, C. Cavallotti and A. Famulari, Phys. Chem. Chem. Phys., 2016, 18, 29616–29628 RSC; (d) L. Catalano, D. P. Karothu, S. Schramm, E. Ahmed, R. Rezgui, T. J. Barber, A. Famulari and P. Naumov, Angew. Chem., Int. Ed., 2018, 57, 17254–17258 CrossRef CAS PubMed; (e) S. Torresi, A. Famulari and J. Martí-Rujas, J. Am. Chem. Soc., 2020, 142, 9537–9543 CrossRef CAS PubMed; (f) J. Marti-Rujas, S. Ma and A. Famulari, Inorg. Chem., 2022, 61, 10863–10871 CrossRef CAS PubMed.
- S. Mahapatra, T. S. Thakur, S. Joseph, S. Varughese and G. R. Desiraju, Cryst. Growth Des., 2010, 10, 3191–3202 CrossRef CAS.
- A. Nangia, Acc. Chem. Res., 2008, 41, 595–604 CrossRef CAS PubMed.
- J. F. Stoddart, Z. Liu, S. T. Schneebeli and J. F. Stoddart, Chimia, 2014, 68, 315–320 CrossRef PubMed.
- J. W. Steed, CrystEngComm, 2003, 5, 169 RSC.
- G. M. Sheldrick, SHELXTL reference manual, Siemens analytical X-ray systems, Inc., Madison, WI, 1996 Search PubMed.
- G. M. Sheldrick, A short history of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Further details on the synthesis and crystallographic information. CCDC 2109973 and 2108276. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ce01174c |
| This journal is © The Royal Society of Chemistry 2022 |