 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
In situ time-resolved monitoring of mixed-ligand metal–organic framework mechanosynthesis†
Max
Rautenberg
ab,
Biswajit
Bhattacharya
*a,
Julia
Witt
 a,
Mohit
Jain
ac and
Franziska
Emmerling
a,
Mohit
Jain
ac and
Franziska
Emmerling
 *ab
*ab
aBAM Federal Institute for Materials Research and Testing, Richard-Willstätter-Str. 11, 12489 Berlin, Germany. E-mail: franziska.emmerling@bam.de
bDepartment of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, 12489 Berlin, Germany
cDepartment of Materials Science, Technical University of Darmstadt, Karolinenplatz 5, 64289 Darmstadt, Germany
First published on 5th September 2022
Abstract
The mechanism of mixed-ligand metal–organic framework (MOF) formation, and the possible role of intermediate single-ligand metal complexes during mechanosynthesis, have not been explored yet. For the first time, we report here in situ real-time monitoring of the mechanochemical formation mechanism of mixed-ligand MOFs. Our results show that binary phases can act as intermediates or competing products in one-pot and stepwise synthesis.
Metal–organic frameworks (MOFs) are crystalline porous materials built from inorganic metal nodes connected with organic ligands resulting in two- or three-dimensional network structures with potential voids.1,2 Thanks to their inherent porosity and large surface area, these materials are ideal candidates in gas storage/separation,3,4 sensing,5,6 and catalysis.7 The unique combination of compositional modularity of MOFs by varying the organic/inorganic subunit has further propelled research interest in these materials.8,9 In this regard, mixed-ligand approaches comprising two different types of ligands with distinct functionalities, such as carboxylate or phosphonate groups combined with a basic N,N′-donor ligand, were introduced as a successful strategy for tuning the physicochemical properties of MOFs.10–12 Despite several advancements, the development of new MOF materials is still typically based on trial-and-error. The investigation of the reaction parameters such as concentration, time, temperature, or solvent conditions required for a desired MOF structure is demanding and needs combinatorial screening. Recently, different spectroscopic and computational approaches have been used to study the crystal nucleation and growth of MOFs.13–16 However, there is still a lack of understanding of the formation mechanism, i.e., how chemical coordination between metal nodes and organic ligands occurs during MOF self-assembly or which fundamental building units govern the MOF synthesis.
In this regard, environmentally benign rapid mechanochemical syntheses provide an opportunity to study the real-time chemical transformations of a wide range of solid molecules and materials including nanomaterials, alloys, organometallic complexes, pharmaceutical cocrystals, and MOFs.17–25 Mechanochemical grinding or milling of solids performed either under solvent-free conditions or using catalytic amounts of solvent can easily be monitored by time-resolved in situ (TRIS) X-ray diffraction or spectroscopy probing the local geometry, phase composition, and crystallinity of intermediates and products.26–30 TRIS X-ray powder diffraction,31–33 Raman spectroscopy,28 and thermography30 or combinations thereof34,35 provided insights into the mechanism of mechanochemical syntheses of MOFs. Very recently, our group demonstrated the accurate measurement of the microstructure of ZIF-8 during ball milling by introducing a new kind of milling jar in time-resolved in situ-XRD measurements.36 Typically, investigations are focused on the synthesis of single ligand MOFs and examples of mixed-ligand MOFs are scarce. The competitive coordination of the metal ions by two different ligands allows to tune the network structures in mixed-ligand MOFs. Different possibilities for the formation of single-component coordination complexes exist along with dual ligand complexes. The question arises as to what role the individual coordination complexes play in the formation of the MOFs with mixed ligands. Thus, the formation mechanism of these materials must be better understood before such materials can be selectively prepared in bulk scale for targeted practical applications.
In this work, we explored the mechanochemical synthesis of two isostructural mixed ligand phosphonate-based proton conductive MOFs,37 {Co(H2PhDPA)(4,4′-bipy)(H2O)·2H2O}n (BAM-1), {Fe(H2PhDPA)(4,4′-bipy)(H2O)·2H2O}n (BAM-2) [where H2PhDPA2− = phenylenediphosphonate and 4,4′-bipy = 4,4′-bipyridine] by using tandem real-time in situ powder X-ray diffraction (PXRD). Along with the one-pot synthesis, the step-by-step reactions where the metal source reacts with one ligand first and subsequently with the second ligand are monitored to extract detailed information on the development of the product structure. The insight gained from these incremental and one-pot syntheses allows us to estimate formation mechanisms for BAM-1 and BAM-2.
Liquid assisted grinding (LAG) of a stoichiometric mixture of Co(OAc)2·4H2O or FeCl2·4H2O and NaHCO3 with 4,4′-bipy and H4PhDPA at 50 Hz for 30 min led to the formation of BAM-1 and BAM-2 (Fig. 1). BAM-1 was obtained as a pure phase, whereas the formation of BAM-2 was not completed even after prolong grinding. A washing step with water was needed to remove the side products from the obtained powder of BAM-2.
The formation mechanism of phosphonate based mixed-ligand MOFs could in principle follow different pathways: i) the metal precursor can react with one of the organic linkers to form a single ligand-based metal complex, which further reacts with the second linker to form the final framework. ii) Alternatively, the simultaneous coordination of both ligands to the metal centre is also a plausible mechanism. To understand which pathway leads to the final MOF and which is favoured, we split the mechanochemical synthesis of BAM-1 and BAM-2 into two two-step reactions, consisting of one ligand offered in each step. The synthesis increments and the one-pot reactions are monitored by in situ PXRD to assess the conversion and formation rates of the reactions and the possible mechanisms.
During the three-component reaction of BAM-1, the early stage of the transformation shows a loss of intensity of the diffraction of starting materials. This could be attributed to an amorphization of the starting materials or the powder getting stuck in the milling vessel. The sudden appearance of reflections at q = 9.1 nm−1 for 15 s and q = 14.3 nm−1 for 10 s suggests that crystalline material is present but does not pass through the beam. After 1 min of milling, reflections of BAM-1 start to grow, and reach a plateau after approximately 2 min (Fig. 2a and S1†). For BAM-2, Bragg reflections from starting materials disappear after 30 s and new reflections grow in the early stage of the reaction. These new reflections (q = 7.61, 10.53, 17.28 nm−1) are consistent with a mixture of FeCl2(4,4′-bipy) and unidentified phases. With continued milling, the reflections of BAM-2 appear. After 30 min of grinding the reflections of BAM-2 reach the maximum intensity, leading to a mixture of BAM-2, FeCl2(4,4′-bipy)38 and an unknown phase, that persist even after 10 h of extensive grinding (Fig. 2b and S2†).
The three-component reactions showed a difference for the two metal atoms (M = Co, Fe). Specifically, the formation of BAM-2 passed through a binary phase intermediate in contrast to BAM-1. We therefore aim to better understand if the difference in reaction pathways relates to the existence of binary phases of both metal complexes, the relative reactivity of the binary phases, or relative kinetics of their formation.
To probe this, we have conducted step by step reactions where the ligands are added sequentially in the reaction vessel (Scheme 1). The TRIS XRD data for milling 4,4′-bipy with Co(OAc)2·4H2O or FeCl2·4H2O (Scheme 1, reactions Ia) show a rapid consumption of the starting material, leading to the formation of {Co(OAc)2(4,4′-bipy)}n (ref. 39) or {FeCl2(4,4′-bipy)}n, respectively. Both reactions were completed within 4 min and 30 s for Co(II) and Fe(II), and no further changes were observed within 30 min of grinding (Fig. 3a and e and S3 and S4†). The subsequent addition of H4-PhDPA (Scheme 1, reactions Ib) led to the conversion of {Co(OAc)2(4,4′-bipy)}n to BAM-1, which was completed after 3 min of grinding (Fig. 3b, and S5†). Whereas, during milling of {FeCl2(4,4′-bipy)}n with H4-PhDPA and NaHCO3, BAM-2 starts to grow with simultaneous consumption of {FeCl2(4,4′-bipy)}n (Fig. 3f and S6†). After 35 min of grinding, approximately 65% conversion to BAM-2 was observed. The incomplete conversion to BAM-2 is consistent with the one-pot synthesis, see Fig. 2b and S2.†
 | ||
| Scheme 1 Three synthesis pathways towards BAM-1 and BAM-2 either as incremental approaches (top, blue, Ia and Ib; or bottom, red IIa and IIb) or direct one-pot approach (middle, black, III). | ||
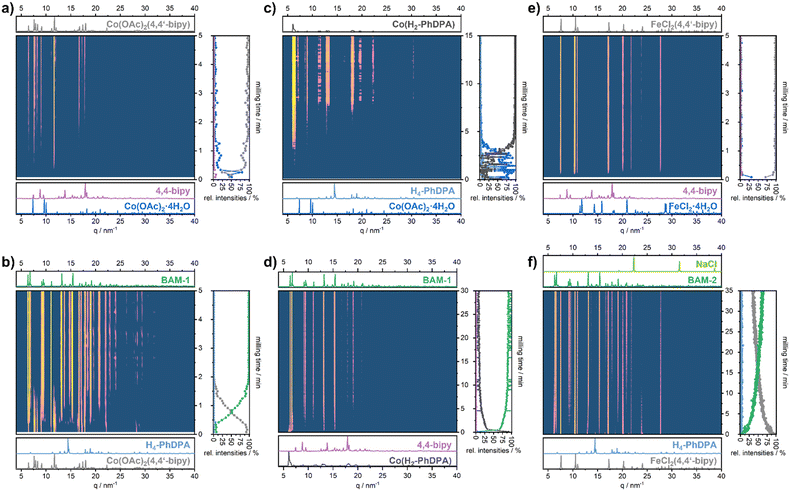 | ||
| Fig. 3 TRIS PXRD data of the mechanochemical reactions Ia (a), Ib (b), IIa (c), and IIb (d) for BAM-1 and reactions Ia (e) and Ib (f) for BAM-2 (see Scheme 1 for reaction overview). | ||
Initially, no crystalline phases were detected in the experiments reversing the order of ligand addition milling Co(OAc)2·4H2O with H4-PhDPA (Scheme 1, reactions IIa, Fig. 3c and S7†). Reflections appear around 4 min and continue to grow, reaching a plateau at 8 min. The reflections could not be assigned to any known crystalline phase, but it can be assumed that mixtures of cobalt–phenylenediphosphonates (e.g. Co(H2PhDPA)) with varying stoichiometry are present (Fig. 3b and S7†). With the addition of 4,4′-bipy (Scheme 1, reactions IIb), the presumed mixture of Co(H2PhDPA) is consumed within the first minute of milling (Fig. 3d and S9†). Finally, the crystallization of BAM-1 starts and continues over the next 14 min. In contrast, co-grinding FeCl2·4H2O with H4-PhDPA, and NaHCO3, yielded reflections of the side product NaCl and another unknow crystalline phase, presumably Fe(H2-PhDPA) (Fig. S8†). We suspect that this is the same unknown phase as observed in the one pot synthesis. Surprisingly, milling this unknown phase with 4,4′-bipy (IIb), did not yield BAM-2, but a complete consumption of NaCl and a partial consumption of 4,4′-bipy can be observed. This can be explained by the formation of a 4,4′-bipy-hydrogen chloride salt or a hydrate thereof (Fig. S10†).
Moreover, we have tested the bulk synthesized BAM-1 and BAM-2 as electrocatalysts for water splitting (see ESI:† Fig. S17). Both compounds exhibit moderate reactivity towards oxygen evolution reaction (OER), which need further investigations for a follow-up work.
Our stepwise synthesis demonstrates that the binary phases can in principle behave as intermediates for the formation of BAM-1. Their absence in the one-pot synthesis can be ascribed to their rapid consumption, consistent to the speed of their reactivity during step-wise synthesis. However, we have shown that BAM-2 can only form via a single step reaction or the binary phase, {FeCl2(4,4′-bipy)}n. The binary phase Fe(H2-PhDPA) is instead a competitive product, and its formation is responsible for the inability to achieve the quantitative yield of BAM-2 during the one-pot synthesis. Our studies reveal the complex behaviour of mechanochemical synthesis of mixed ligand metal organic frameworks. We expect further investigation in these directions will provide exciting insights into the mechanisms of materials synthesis by environmentally benign ways.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Helmholtz-Zentrum Berlin für Materialien und Energie for the allocation of synchrotron radiation beamtime. Gefördert durch die Deutsche Forschungsgemeinschaft (DFG) – Projektnummer 387284271 – SFB 1349. Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 387284271 – SFB 1349.Notes and references
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. Paik Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 CrossRef CAS.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS.
- R.-B. Lin, S. Xiang, W. Zhou and B. Chen, Chem, 2020, 6, 337–363 CAS.
- K.-J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q.-Y. Zhang and M. J. Zaworotko, Science, 2019, 366, 241–246 CrossRef CAS PubMed.
- B. Bhattacharya, A. Halder, L. Paul, S. Chakrabarti and D. Ghoshal, Chem. – Eur. J., 2016, 22, 14998–15005 CrossRef CAS PubMed.
- E. A. Dolgopolova, A. M. Rice, C. R. Martin and N. B. Shustova, Chem. Soc. Rev., 2018, 47, 4710–4728 RSC.
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev., 2020, 120, 8468–8535 CrossRef CAS PubMed.
- E. A. Dolgopolova, O. A. Ejegbavwo, C. R. Martin, M. D. Smith, W. Setyawan, S. G. Karakalos, C. H. Henager, H.-C. zur Loye and N. B. Shustova, J. Am. Chem. Soc., 2017, 139, 16852–16861 CrossRef CAS.
- J. B. Bailey, L. Zhang, J. A. Chiong, S. Ahn and F. A. Tezcan, J. Am. Chem. Soc., 2017, 139, 8160–8166 CrossRef CAS.
- B. Bhattacharya and D. Ghoshal, CrystEngComm, 2015, 17, 8388–8413 RSC.
- S. Pullen and G. H. Clever, Acc. Chem. Res., 2018, 51, 3052–3064 CrossRef CAS PubMed.
- M. Du, C.-P. Li, C.-S. Liu and S.-M. Fang, Coord. Chem. Rev., 2013, 257, 1282–1305 CrossRef CAS.
- M. J. Van Vleet, T. Weng, X. Li and J. R. Schmidt, Chem. Rev., 2018, 118, 3681–3721 CrossRef CAS PubMed.
- T. D. Petersen, G. Balakrishnan and C. L. Weeks, Dalton Trans., 2015, 44, 12824–12831 RSC.
- S. Hermes, T. Witte, T. Hikov, D. Zacher, S. Bahnmüller, G. Langstein, K. Huber and R. A. Fischer, J. Am. Chem. Soc., 2007, 129, 5324–5325 CrossRef CAS.
- F. Millange, M. I. Medina, N. Guillou, G. Férey, K. M. Golden and R. I. Walton, Angew. Chem., Int. Ed., 2010, 49, 763–766 CrossRef CAS PubMed.
- A. A. L. Michalchuk, E. V. Boldyreva, A. M. Belenguer, F. Emmerling and V. V. Boldyrev, Front. Chem., 2021, 9, 685789 CrossRef CAS PubMed.
- T. Stolar and K. Užarević, CrystEngComm, 2020, 22, 4511–4525 RSC.
- L. Tröbs and F. Emmerling, Faraday Discuss., 2014, 170, 109–119 RSC.
- V. Shivam, J. Basu, Y. Shadangi, M. K. Singh and N. K. Mukhopadhyay, J. Alloys Compd., 2018, 757, 87–97 CrossRef CAS.
- P. F. M. de Oliveira, A. A. L. Michalchuk, A. G. Buzanich, R. Bienert, R. M. Torresi, P. H. C. Camargo and F. Emmerling, Chem. Commun., 2020, 56, 10329–10332 RSC.
- H. Kulla, A. A. L. Michalchuk and F. Emmerling, Chem. Commun., 2019, 55, 9793–9796 RSC.
- M. Klimakow, P. Klobes, A. F. Thunemann, K. Rademann and F. Emmerling, Chem. Mater., 2010, 22, 5216–5221 CrossRef CAS.
- A. L. Garay, A. Pichon and S. L. James, Chem. Soc. Rev., 2007, 36, 846–855 RSC.
- A. Pichon, A. Lazuen-Garay and S. L. James, CrystEngComm, 2006, 8, 211–214 RSC.
- T. Friščić, I. Halasz, P. J. Beldon, A. M. Belenguer, F. Adams, S. A. J. Kimber, V. Honkimäki and R. E. Dinnebier, Nat. Chem., 2013, 5, 66–73 CrossRef PubMed.
- A. A. L. Michalchuk and F. Emmerling, Angew. Chem., Int. Ed., 2022, 61(21), e202117270 CrossRef CAS.
- S. Lukin, K. Užarević and I. Halasz, Nat. Protoc., 2021, 16, 3492–3521 CrossRef CAS PubMed.
- I. Sović, S. Lukin, E. Meštrović, I. Halasz, A. Porcheddu, F. Delogu, P. C. Ricci, F. Caron, T. Perilli, A. Dogan and E. Colacino, ACS Omega, 2020, 5, 28663–28672 CrossRef PubMed.
- H. Kulla, M. Wilke, F. Fischer, M. Röllig, C. Maierhofer and F. Emmerling, Chem. Commun., 2017, 53, 1664–1667 RSC.
- T. Stolar, L. Batzdorf, S. Lukin, D. Žilić, C. Motillo, T. Friščić, F. Emmerling, I. Halasz and K. Užarević, Inorg. Chem., 2017, 56, 6599–6608 CrossRef CAS PubMed.
- L. S. Germann, A. D. Katsenis, I. Huskić, P. A. Julien, K. Užarević, M. Etter, O. K. Farha, T. Friščić and R. E. Dinnebier, Cryst. Growth Des., 2020, 20, 49–54 CrossRef CAS.
- J. Beamish-Cook, K. Shankland, C. A. Murray and P. Vaqueiro, Cryst. Growth Des., 2021, 21, 3047–3055 CrossRef CAS PubMed.
- L. Batzdorf, F. Fischer, M. Wilke, K.-J. Wenzel and F. Emmerling, Angew. Chem., 2015, 127, 1819–1822 CrossRef.
- H. Kulla, S. Haferkamp, I. Akhmetova, M. Röllig, C. Maierhofer, K. Rademann and F. Emmerling, Angew. Chem., Int. Ed., 2018, 57, 5930–5933 CrossRef CAS PubMed.
- G. I. Lampronti, A. A. L. Michalchuk, P. P. Mazzeo, A. M. Belenguer, J. K. M. Sanders, A. Bacchi and F. Emmerling, Nat. Commun., 2021, 12, 6134 CrossRef CAS PubMed.
- M. Rautenberg, B. Bhattacharya, C. Das and F. Emmerling, Inorg. Chem., 2022, 61, 10801–10809 CrossRef CAS PubMed.
- M. A. Lawandy, X. Huang, R.-J. Wang, J. Li, J. Y. Lu, T. Yuen and C. L. Lin, Inorg. Chem., 1999, 38, 5410–5414 CrossRef CAS.
- J. Lu, C. Yu, T. Niu, T. Paliwala, G. Crisci, F. Somosa and A. J. Jacobson, Inorg. Chem., 1998, 37, 4637–4640 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Time-resolved in situ XRD data, Raman data and electrochemical measurements. See DOI: https://doi.org/10.1039/d2ce00803c |
| This journal is © The Royal Society of Chemistry 2022 |


