Three new POM-based coordination polymers with 1,3,5-tris(1-imidazolyl)benzene ligand: syntheses, structures and proton conductivity†
Abstract
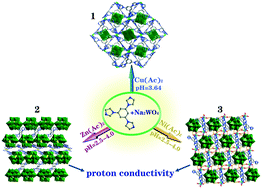
Three new POM-based coordination polymers, [Cu(timb)2(W6O19)]n (1), {[Zn3(Htimb)2(H2timb)2(H2O)2(ZnW12O40)2]·2H2O}n (2), and {[Ni(Htimb)(H2O)3(H2W12O40)0.5]·3H2O}n (3) (timb = 1,3,5-tris(1-imidazolyl)benzene), have been prepared under hydrothermal conditions. Compound 1 contains one 3D framework constructed by CuII atoms and timb ligands with a rare 3,6-connected pyr topology, while the polyoxoanions are situated in the cavities. For compound 2, the 3D framework is composed of a 2,4-connected network (A) and hcb network (B) in the BBABB mode. The 1D chains of compound 3 are arranged into a 3D framework by a variety of hydrogen bonding interactions between the [H2W12O40]6− polyoxoanions, coordinated water molecules and free water molecules. Magnetic data of compounds 1 and 3 show the presence of antiferromagnetic behavior. In addition, the highest proton conductivities of compounds 2 and 3 reach 2.32 × 10−5 S cm−1 and 3.38 × 10−5 S cm−1 at 358 K and 98% RH, respectively.


 Please wait while we load your content...
Please wait while we load your content...