Syntheses, structures and optical properties of two B3O7 cluster-based borates†
Abstract
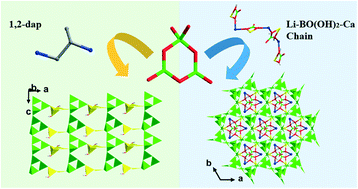
Two new B3O7 cluster-based borates, [B6O9(OH)2]·H2dap (1, dap = 1,2-diaminopropane) and Li0.5Ca0.5[B3O5][BO(OH)2]0.5 (2), were solvothermally synthesized using different organic and inorganic templates, respectively. In 1, two different B3O7 clusters interconnect to construct a rare 2D fluctuant layer with 11-MR unclosed channels. Templating [H21,2dap]2+ located in the interlayers stabilizes the layers through H-bonds. 2 features an acentric 3D open framework with 17-MR large channels built by 4-connected B3O7 clusters. Guest Li-BO(OH)2-Ca 1D chains intercalate in the 17-MR channels, directing the formation of the 3D open framework through host–guest symmetry and charge matching. 2 is a phase-matchable nonlinear optical (NLO) material with a second-harmonic generation (SHG) response ∼1.6 times that of KDP (KH2PO4). UV-vis absorption spectra reveal that 1 and 2 have short cut-off edges of 205 and 213 nm, respectively, indicating that 1 is a wide-band semiconductor and 2 is a UV NLO material.


 Please wait while we load your content...
Please wait while we load your content...