Polymorphism and cocrystal salt formation of 2-((2,6-dichlorophenyl)amino)benzoic acid, harvest of a second form of 2-((2,6-dimethylphenyl)amino)benzoic acid, and isomorphism between the two systems†
Abstract
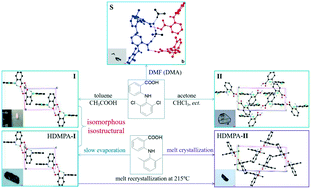
2-((2,6-Dichlorophenyl)amino)benzoic acid (2-DCABA), a potential non-steroidal anti-inflammatory drug and an analog of 2-((2,6-dimethylphenyl)amino)benzoic acid (HDMPA) was synthesized and its polymorphism was studied to investigate the effect of double Cl–CH3 exchange. Three forms-two polymorphs (I and II) and one cocrystal salt (S)-were obtained through polymorph screening in a variety of solvents, and the elusive single crystals of form II of HDMPA were harvested through melt crystallization. The crystal forms were characterized by single-crystal X-ray diffraction, powder X-ray diffraction (PXRD), DSC, and FT-IR. Isomorphism and isostructurality were observed between the form I of each system. The polymorphism of 2-DCABA seems to stem from the conformational flexibility of the molecule, similar to that of HDMPA. Hirshfeld analysis revealed different intermolecular interactions contributing to the stability of the crystal form I of 2-DCABA and HDMPA-I, despite their structural similarity.


 Please wait while we load your content...
Please wait while we load your content...