A facile method to enhance the output performance of triboelectric nanogenerators based on coordination polymers by modulating terminal coordination groups†
Wenjie
Wang
,
Ying-Ying
Zhang
 *,
Sheng
Zhang
,
Chao
Huang
*,
Sheng
Zhang
,
Chao
Huang
 * and
Liwei
Mi
* and
Liwei
Mi
 *
*
Center for Advanced Materials Research, Henan Key Laboratory of Functional Salt Materials, Zhongyuan University of Technology, Zhengzhou, 450007, P. R. China. E-mail: 6477@zut.edu.cn; huangchao@zut.edu.cn; mlwzzu@163.com
First published on 10th November 2021
Abstract
The output performance of triboelectric nanogenerators (TENGs) basically depends on the inherent nature of friction electrode materials. In this work, a series of isostructural Cu(I)-CPs (coordination polymers) with different terminal coordination groups from the same main group, formulated as [Cu(μ3-H3ttc)X]n (1: X = Cl, 2: X = Br and 3: X = I; H3ttc = trithiocyanuric acid), were chosen as the friction electrode materials to fabricate TENGs (Cl-, Br- and I-TENG based on compounds 1–3, respectively) to clearly clarify the influence of chemical microcomposition on the output performance of TENGs. The results indicated that the polarity of the compounds determined from dielectric constants was greatly affected by the halogen elements, which plays a decisive role in the output performance of the TENGs. I-TENG based on the Cu(I)-CP containing the least electronegative halogen atoms but with the largest polarity had the largest output performance, followed by Br- and Cl-TENG. As a practical application, I-TENG was used as a power source to construct a self-powered anticorrosion system to protect metal materials from corrosion.
Introduction
Due to the depletion of fossil fuels and the intensification of environmental pollution, developing sustainable clean energy sources is urgently needed. Harvesting energy from the surrounding environment has been proven to be one of the most promising ways to alleviate energy shortage.1,2 In 2012, triboelectric nanogenerators (TENGs) were invented as a new type of energy collection device by Wang and co-workers3 and can directly convert various types of mechanical energy into electrical energy,3–5 including but not limited to human motion,6 vibrational energy,7 wind energy,8 water wave energy,9 air flow energy,10 raindrops11 and sound energy.12 They have attracted extensive attention due to their simple structure, high efficiency and low cost during the past few years. Along with the unceasing research of TENGs, it was found that the output performance of TENGs could be significantly improved by optimization of the device structure, the friction movement mode, and the properties of friction electrode materials.13–16 Of all the factors mentioned above, the intrinsic nature of triboelectric materials (i.e., the ability to gain and lose electrons and polarity) is the most critical factor, but it is usually limited to some polymers (e.g. polyamide, Kapton, polydimethylsiloxane) and a few metals (e.g. aluminum, nickel) in the triboelectric series.17Coordination polymers (CPs) are a fascinating type of porous crystalline material constructed by the coordination of metal ions and organic ligands.18,19 The diversity of CP or MOF materials assembled from a variety of metal centers and organic ligands has led to their wide applications in the fields of catalysis,20 gas storage,21,22 sensors,23 carbon capture,24 and energy storage.25 Large specific surface areas, easy modification and adjustable structures26–34 are some of the essential characteristics of CPs. To overcome the shortcomings of conventional materials from the triboelectric series, such as difficulty in accurate modification and functionalization, CPs or metal–organic frameworks (MOFs) have been introduced into the fabrication of TENG devices as electrode materials.35–40 CP- or MOF-based TENGs have shown good output performance, which could be enhanced by tuning the content of the active materials, the metal nodes and the organic ligands. However, except for the approach of regulating the content in the nanocomposite film,35 the reported strategies for the improvement of the TENG's output accompanied with the changes of CPs or MOFs structures, which might also result in the changes of the output performance. Therefore, it is of great importance to study a series of CPs or MOFs without changing their structure to further investigate the relationship between the structure and performance and, to some extent, provide a facile and effective method for the improvement of the output performance of CP-based TENGs for further applications.
Herein, we selected a series of isostructural Cu(I)-CPs composed of the same metal center Cu(I) and ligand trithiocyanuric acid (H3ttc), formulated as [Cu(μ3-H3ttc)X]n (1: X = Cl, 2: X = Br and 3: X = I), as triboelectric materials to fabricate TENGs (Cl-, Br- and I-TENG based on compounds 1–3, respectively) to investigate the effect of the different coordinated terminal groups with distinct electronegativity on the output performance of the TENGs. Electrochemical characterization showed that the output performance of I-TENG was the largest, followed by that of Br- and Cl-TENG. In addition, it was introduced into a self-powered anticorrosion system to protect metal materials from corrosion.
Experimental section
Materials and physical measurements
All the chemical reagents in this work were obtained from commercial suppliers and can be used without further purification. Compounds 1–3 were prepared according to a modified procedure reported in the literature.41 The characterization data for compounds 1–3 and the CP-based TENGs were collected using the corresponding instruments. The electrochemical measurements of carbon steel with and without I-TENG were carried out by using an electrochemical workstation (CHI 660E, Shanghai Chenhua Instrument Co., Ltd, China) in a three-electrode system (see the ESI† for details).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The test tube was sealed with a preservative film and allowed to stand at room temperature for 5 days to obtain orange red bulk crystals. Yield: 43% based on CuCl. Elemental analysis calcd. (%) for Cu3C3N3S3Cl3: C, 13.04; H, 1.09; N, 15.21. Found: C, 13.03; H, 1.08; N, 15.23. IR (KBr pellets, ν/cm−1): 3448(s), 2857(w), 1529(s), 1388(m), 1238(w), 1129(s), 806(w), 597(w), 462(w).
1). The test tube was sealed with a preservative film and allowed to stand at room temperature for 5 days to obtain orange red bulk crystals. Yield: 43% based on CuCl. Elemental analysis calcd. (%) for Cu3C3N3S3Cl3: C, 13.04; H, 1.09; N, 15.21. Found: C, 13.03; H, 1.08; N, 15.23. IR (KBr pellets, ν/cm−1): 3448(s), 2857(w), 1529(s), 1388(m), 1238(w), 1129(s), 806(w), 597(w), 462(w).
Results and discussion
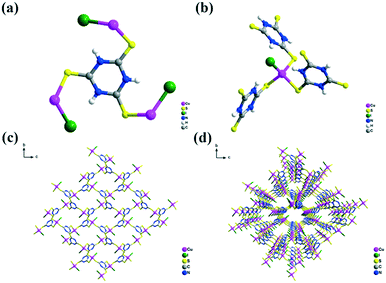
Trithiocyanuric acid (H3ttc), which can exist in four tautomeric and resonance structures (Scheme S1†), was considered as a multifunctional bridging ligand to ligate metal ions in either deprotonated (H2ttc−, Httc2−, and ttc3−) or neutral (H3ttc) form. [Cu(μ3-H3ttc)X]n (1: X = Cl, 2: X = Br and 3: X = I) were obtained by the slow diffusion of the solution of the organic sulfur ligand (H3ttc) in CH2Cl2 into the solution of CuCl, CuBr, and CuI in CH3CN at room temperature, respectively. Compounds 1–3 were reported as 2-fold interpenetrating three-dimensional (3D) networks with neutral H3ttc ligands.41 They are isostructural but have different terminal coordination groups (Fig. S1, S2† and 1). Each ligand is essentially coplanar and connected with three Cu(I) ions through bridging S atoms (Fig. S1a, S2a† and 1a). The central metal Cu(I) is in an almost typical tetrahedral coordination configuration with a τ4 parameter of 1.00 (ref. 44) completed by three S atoms from three separate H3ttc ligands and one halogen atom (Fig. S1b, S2b† and 1b), generating a series of 3D structures (Fig. S1d, S2d† and 1d).The powder X-ray diffraction (PXRD) patterns of compounds 1–3 are consistent with the simulated ones from reported single-crystal data,41 verifying their purity (Fig. S3a–c†). The peaks in the regions of 3440–3448 cm−1 and 1120–1129 cm−1 in the Fourier transform infrared (FT-IR) spectra of compounds 1–3 (Fig. S3d–f†) could be assigned to the N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S groups, respectively. In addition, X-ray photoelectron spectroscopy (XPS) of the as-prepared compounds 1–3 was carried out to determine the valence state of Cu and the existence of the different halogen atoms (Fig. S4†). The peaks of Cu 2p3/2 and Cu 2p1/2 at 932.5 eV and 952.2 eV proved that Cu exists in the form of Cu+ (Fig. S4b†). In Fig. S4c,† two binding energy peaks, 197.73 eV and 199.18 eV, correspond to Cl 2p3/2 and Cl 2p1/2, respectively. The characteristic peak at 68.5 eV of compound 2 can be assigned to Br 3d5/2 (Fig. S4d†), while the peaks at 619.73 eV and 630.68 eV belong to I 3p5/2 and I 3p3/2 of I (Fig. S4e†).
S groups, respectively. In addition, X-ray photoelectron spectroscopy (XPS) of the as-prepared compounds 1–3 was carried out to determine the valence state of Cu and the existence of the different halogen atoms (Fig. S4†). The peaks of Cu 2p3/2 and Cu 2p1/2 at 932.5 eV and 952.2 eV proved that Cu exists in the form of Cu+ (Fig. S4b†). In Fig. S4c,† two binding energy peaks, 197.73 eV and 199.18 eV, correspond to Cl 2p3/2 and Cl 2p1/2, respectively. The characteristic peak at 68.5 eV of compound 2 can be assigned to Br 3d5/2 (Fig. S4d†), while the peaks at 619.73 eV and 630.68 eV belong to I 3p5/2 and I 3p3/2 of I (Fig. S4e†).
Considering that compounds 1–3 were assembled using identical organic ligands and metal ions but have different terminal coordination groups (Cl, Br, and I) from the same main group with different electronegativities or electron-attracting abilities, we are curious about the effect of changes in the chemical microcomposition on the output performance of the TENGs. Generally, the nature of friction pair materials (i.e., the polarity, shape and size) plays an important role in the output performance of TENGs.45,46 Therefore, the dielectric constants of 1–3 were measured (Fig. S5†) in the frequency range of 103–107 Hz at room temperature to determine their polarities. Obviously, the dielectric constant of compound 3 is larger than those of 1 and 2, which might result in I-TENG producing a higher output than Br-TENG and Cl-TENG.
In addition, nano- or microscale materials with large specific surface areas can make better contact with the counter electrode in the friction process and improve the electron density.39,40,46 Therefore, the bulk crystal samples of the crystalline compounds 1–3 were fully ground into powder and evenly coated on a copper strip to improve the output performance of the TENGs. In the coating process, the same amount of compounds 1–3 was used to fabricate active friction layers, resulting in similar thicknesses of triboelectric materials (Fig. S6†) to eliminate the influence of the film thickness on the final output performance.
The 3D structure of the CP-TENGs is shown in Fig. S7i.† The operating mode of the CP-TENGs is a vertical contact-separation mode with CPs and polyvinylidene fluoride (PVDF) as the positive and negative materials, respectively. The working principle of TENGs is based on the coupling effect of triboelectrification and electrostatic induction during the contact process of friction electrode materials with opposite triboelectric polarities (see the ESI†).47
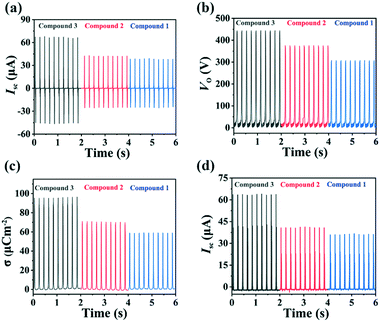
The fabricated TENGs were exerted the external force through the periodic movement of a linear motor to imitate the mechanical energy in the real environment. Under the same experimental conditions, the short circuit current (Isc) and output voltage (Vo) of Cl-, Br- and I-TENG at 5 Hz were obtained with values of 41.3 μA and 305 V, 44.5 μA and 375 V, and 69.4 μA and 445 V, respectively (Fig. 2a and b). It is obvious that both the Isc and Vo values of I-TENG are the highest. Charge density (σ), as a standard for evaluating the material availability, is also an essential parameter to evaluate the properties of TENGs.48–50 The σ values of Cl-, Br- and I-TENG are approximately 59.3, 70 and 97.5 μC m−2, respectively (Fig. 2c). As expected, the output of I-TENG is the largest, which is consistent with the molecular polarity, as confirmed from the dielectric constants. To sum up, the output performance of the prepared TENGs based on Cu(I)-CPs followed the order I-TENG > Br-TENG > Cl-TENG, which is opposite to that of the electronegativity of the halogens (I < Br < Cl). This further confirmed that the output performance of TENGs is closely related to small changes in the structures of the electrode materials. As a comparative study, TENGs based on the pure organic ligand H3ttc, the inorganic compound CuI, and their mixture in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were assembled and denoted as H3ttc-TENG, CuI-TENG and Mix-TENG, respectively. Both the Isc and Vo values for these TENGs were measured under the same experimental conditions. As shown in Fig. S8,† it can be observed that the output performance followed the trend H3ttc-TENG > Mix-TENG > CuI-TENG, suggesting that the introduction of the organic ligands greatly increases the performance of the inorganic compounds. However, when iodine was used for the fabrication of TENGs to evaluate its electron donor and acceptor properties, we found that it was hard to evenly spread onto the Cu tape with the same thickness as the other compounds after grinding or crushing, and this friction layer easily adhered to the opposite layer because of the gradual evaporation of iodine in the friction process (Fig. S9†).
1 were assembled and denoted as H3ttc-TENG, CuI-TENG and Mix-TENG, respectively. Both the Isc and Vo values for these TENGs were measured under the same experimental conditions. As shown in Fig. S8,† it can be observed that the output performance followed the trend H3ttc-TENG > Mix-TENG > CuI-TENG, suggesting that the introduction of the organic ligands greatly increases the performance of the inorganic compounds. However, when iodine was used for the fabrication of TENGs to evaluate its electron donor and acceptor properties, we found that it was hard to evenly spread onto the Cu tape with the same thickness as the other compounds after grinding or crushing, and this friction layer easily adhered to the opposite layer because of the gradual evaporation of iodine in the friction process (Fig. S9†).
 | ||
| Fig. 2 The output performances of the TENGs based on compounds 1–3: (a) Isc, (b) Vo, (c) σ and (d) the rectified Isc, respectively. | ||
Therefore, it was difficult to precisely investigate the performance of the TENGs in the next step.
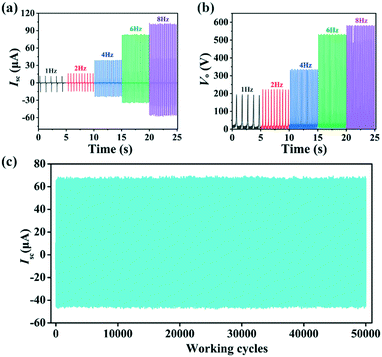
For practical applications, the stability and durability of I-TENG were tested at a frequency of 5 Hz. The cycling experiment demonstrated that both the Isc and Vo values of I-TENG displayed no noticeable change even after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 3c and S10†), indicating excellent stability. Moreover, the Isc and Vo values of I-TENG at 1 Hz, 2 Hz, 4 Hz, 6 Hz and 8 Hz were measured to explore the influence of the test frequency on the output performance of the TENGs (Fig. 3a and b). Both the Isc and Vo values increased as the operating frequency increased, with maximum values of up to 104 μA and 587 V at 8 Hz, respectively. To further verify the stability of the triboelectric materials, we observed the morphology of compounds 1–3 and PVDF by scanning electron microscopy (SEM) and the elemental distribution of compounds 1–3 by energy-dispersive X-ray spectroscopy (EDS). As shown in Fig. S11–S14,† the morphology of compounds 1–3 showed almost no change before and after the test, indicating that compounds 1–3 were relatively stable.
000 cycles (Fig. 3c and S10†), indicating excellent stability. Moreover, the Isc and Vo values of I-TENG at 1 Hz, 2 Hz, 4 Hz, 6 Hz and 8 Hz were measured to explore the influence of the test frequency on the output performance of the TENGs (Fig. 3a and b). Both the Isc and Vo values increased as the operating frequency increased, with maximum values of up to 104 μA and 587 V at 8 Hz, respectively. To further verify the stability of the triboelectric materials, we observed the morphology of compounds 1–3 and PVDF by scanning electron microscopy (SEM) and the elemental distribution of compounds 1–3 by energy-dispersive X-ray spectroscopy (EDS). As shown in Fig. S11–S14,† the morphology of compounds 1–3 showed almost no change before and after the test, indicating that compounds 1–3 were relatively stable.
Fig. S15a† shows the power density and current under different load resistances. The peak value of the instantaneous power (2060.82 MW m−2) emerges when the load resistance reaches 5 MΩ. In order to demonstrate that I-TENG is suitable for practical applications, various capacitors (0.22, 1, 2.2 and 100 μF) were charged up to 5.5 V with the generated output of the aligned I-TENG at 5 Hz (Fig. S15b†). Fig. S15c† shows the three charge–discharge cycles for a 100 μF capacitor.
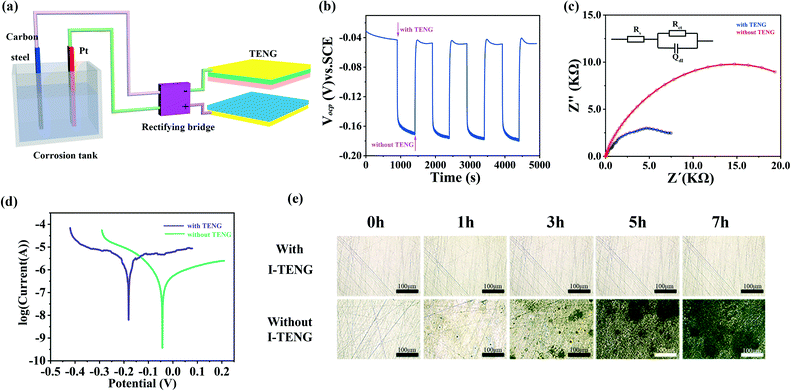
Protecting metals from corrosion is significant and meaningful in our daily life. Cathodic protection is the most commonly used electrochemical protection method for metal corrosion protection.51 However, this protection method is usually accompanied by high costs and severe environmental pollution. As one of the applications of TENGs, self-driving anticorrosion is recognized as a clean, pollution-free, and cost-effective approach,52 and can generate energy by collecting external mechanical energy. To date, it has been successfully applied in cathodic anti-corrosion, self-powered systems and power supplies of portable electronic equipment.53 The principle of impressed current cathodic protection is to use the protected metal and another additional electrode as the two electrodes of the electrolytic cell. The protected metal acts as the cathode and is protected under the action of the applied direct current. Based on the high stability and superior output performance of I-TENG, it is expected to be applied for impressed current cathodic protection of carbon steel. A schematic of the mechanism is presented in Fig. 4a. The output signal of I-TENG was rectified by connecting a rectifier bridge. The positive pole of the rectifier bridge was connected to a platinum electrode while the negative pole was connected to the carbon steel.
Rust is used to visually demonstrate the extent of metal corrosion. We observed the surface of the immersed carbon steel connected with and without I-TENG for 1 h, 3 h, 5 h, and 7 h in a simulated seawater electrolyte based on a 3.5% NaCl solution. It was found that the surface of carbon steel connected with I-TENG had almost no rust spots even after 7 h. However, the surface of carbon steel without I-TENG had an increasing number of rust spots with time (metallographic microscope, Fig. 4e). Furthermore, the EDS spectra showed that the oxygen content of the corrosion products on the surface of the carbon steel with I-TENG protection is much lower than that without I-TENG (Fig. S16†), indicating that the protected carbon steel has less rust, which also verifies the effectivity of cathodic protection.
Open circuit potential (OCP), electrochemical impedance spectroscopy (EIS) and electrochemical polarization curves with or without I-TENG were used to evaluate the characteristics, corrosion degree and cathodic protection efficiency, respectively. The OCP of the protected carbon steel is −0.043 V when it was not connected to I-TENG. When connected to I-TENG, the OCP of the protected carbon steel is −0.181 V. The negative shift of the OCP is caused by the charge transfer between the specimen and the solution, and also indicates that the carbon steel is in a protected state (the greater the absolute value of the negative OCP, the better the cathodic protection effect51). The OCP value moved to the original position again when I-TENG was removed (Fig. 4b).
EIS is another critical technique for evaluating the corrosion behaviour. Fig. 4c shows the impedance spectra of the connected and unconnected I-TENG. Compared with that of the carbon steel connected with I-TENG, the internal resistance of the carbon steel without I-TENG is much larger (the radius of the arc indicates the charge transfer in the electrochemical reaction). The smaller the arc radius, the lower the resistance of the reaction, and the easier the electrochemical reaction occurs on the metal surface, thus protecting the carbon steel. The generated electrons transfer from I-TENG to the carbon steel surface, accelerating the charge transfer between the specimen and the solution interface. The results showed that the carbon steel with I-TENG is more prone to electrochemical reactions than that without I-TENG. We selected the corresponding equivalent circuit diagram (as illustrated in Fig. 4c) according to the impedance fitting process for more directly explaining the impedance map, and the corresponding parameters are shown in Table 1 (Rs: solution resistance, Rct: charge-transfer resistance, Qdl: double-layer resistance).
| R s (Ω) | Q dl | R ct (Ω) | ||
|---|---|---|---|---|
| Y (s·sec^n) | n | |||
| With TENG | 7.754 | 2.560 × 10−4 | 0.7080 | 8953 |
| Without TENG | 9.03 | 1.729 × 10−4 | 0.7591 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 482 482 |
At the same time, we collected the polarization curves of the carbon steel with and without I-TENG. As shown in Fig. 4d, the polarization potential (Ecorr) of the carbon steel with I-TENG negatively shifted compared with that of the carbon steel without I-TENG, which is consistent with the OCP test results. However, the polarization current (Icorr) is the opposite, because the electrons generated by the TENGs are injected into the surface of the carbon steel, strengthening the electrochemical reaction.47Table 2 shows the corresponding values of the electrochemical parameters analyzed from the polarization curves, including Ecorr, Icorr, the anodic Tafel slope (βa) and the cathodic Tafel slope (βc).
| E corr (V(vs. SCE)) | I corr (μA) | −βc (mV dec−1) | β a (mV dec−1) | |
|---|---|---|---|---|
| With TENGs | −0.181 | 15.46 | 2.023 | 0.479 |
| Without TENGs | −0.043 | 0.543 | 8.684 | 4.143 |
Conclusions
In summary, we introduced a series of Cu(I)-CPs with similar structures but with different terminal coordination groups from the same main group into TENG devices, all of which were used as friction electrode materials to fabricate TENGs. The electron gain and loss abilities of the friction electrode materials were evaluated based on the output performance of the TENGs. I-TENG containing the least electronegative halogen atoms exhibited the best output performance, further demonstrating the most robust electron-donating ability. In practical applications, based on the high stability and superior output performance of I-TENG, it was used as a self-powered anti-corrosion device to protect metal materials from corrosion. Therefore, this study not only shows the influence of the chemical microcomposition of friction electrode materials on the output performance of TENGs, but also provides a facile method for designing novel electrode materials to improve the output performance of TENGs in the future.Author contributions
Wenjie Wang: investigation; methodology; formal analysis; writing – original draft. Ying-Ying Zhang: conceptualization; project administration; supervision; writing – review & editing. Sheng Zhang: data curation; validation. Chao Huang: resources; visualization; supervision; writing – review & editing. Liwei Mi: resources; supervision; funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21601212, 21701201, U1804126 and 21671205), the Centaline Science and Technology Innovation Leading Talents Support Plan (204200510014), the Program for Science&Technology Innovation Talents in Universities of Henan Province (22HASTIT030), and the Program for Interdisciplinary Direction Team in Zhongyuan University of Technology.Notes and references
- Z. Zhang, Y. Huang, K. Liu, L. Guo, Q. Yuan and B. Dong, Adv. Mater., 2015, 27, 5906–5914 CrossRef CAS.
- Y. Zhou, P. Luckow, S. J. Smith and L. Clarke, Environ. Sci. Technol., 2012, 46, 7857–7864 CrossRef CAS PubMed.
- F.-R. Fan, Z.-Q. Tian and Z. L. Wang, Nano Energy, 2012, 1, 328–334 CrossRef CAS.
- Z. Liu, H. Li, B. Shi, Y. Fan, Z. L. Wang and Z. Li, Adv. Funct. Mater., 2019, 29, 1808820 CrossRef.
- S. Korkmaz and İ. A. Kariper, Nano Energy, 2021, 84, 105888 CrossRef CAS.
- V. M. H. Ng, L. B. Kong, W. Que, C. Wang, S. Li and T. Zhang, Compr. Energy Syst., 2018, 2, 720–759 CAS.
- L. Wang, T. He, Z. Zhang, L. Zhao, C. Lee, G. Luo, Q. Mao, P. Yang, Q. Lin, X. Li, R. Maeda and Z. Jiang, Nano Energy, 2021, 80, 105555 CrossRef CAS.
- A. R. Mule, B. Dudem, S. A. Graham and J. S. Yu, Adv. Funct. Mater., 2019, 29, 1807779 CrossRef.
- Z. Saadatnia, E. Asadi, H. Askari, J. Zu and E. Esmailzadeh, Int. J. Energy Res., 2017, 41, 2392–2402 CrossRef.
- J. Wang, P. Liu, C. Meng, H. S. Kwok and Y. Zi, ACS Appl. Mater. Interfaces, 2021, 13, 21450–21458 CrossRef CAS PubMed.
- X. Liu, A. Yu, A. Qin and J. Zhai, Adv. Mater. Technol., 2019, 4, 1900608 CrossRef.
- J. Liu, N. Cui, L. Gu, X. Chen, S. Bai, Y. Zheng, C. Hu and Y. Qin, Nanoscale, 2016, 8, 4938–4944 RSC.
- Z. Quan, C. Han, T. Jiang and Z. L. Wang, Adv. Energy Mater., 2016, 6, 1501799 CrossRef.
- G. Khandelwal, N. P. M. J. Rai and S.-J. Kim, J. Mater. Chem., 2020, 8, 17817–17825 RSC.
- S. Ojha, S. Paria, S. K. Karan, S. K. Si, A. Maitra, A. K. Das, L. Halder, A. Bera, A. De and B. B. Khatua, Nanoscale, 2019, 11, 22989–22999 RSC.
- Y. Wang, M. Chao, P. Wan and L. Zhang, Nano Energy, 2020, 70, 104560 CrossRef CAS.
- Z. L. Wang, ACS Nano, 2013, 7, 9533–9557 CrossRef CAS PubMed.
- M. Y. Masoomi, A. Morsali, A. Dhakshinamoorthy and H. Garcia, Angew. Chem., Int. Ed., 2019, 58, 15188–15205 CrossRef CAS.
- Y. Gu, Y.-N. Wu, L. Li, W. Chen, F. Li and S. Kitagawa, Angew. Chem., Int. Ed., 2017, 56, 15658–15662 CrossRef CAS PubMed.
- J. Gascon, A. Corma, F. Kapteijn and F. X. L. Xamena, ACS Catal., 2013, 4, 361–378 CrossRef.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- J. A. Mason, M. Veenstrab and J. R. Long, Chem. Sci., 2014, 5, 32–51 RSC.
- Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS PubMed.
- D. Farrusseng, C. Daniel, C. Hamill, J. Casaban, T. Didriksen, R. Blom, A. Velte, G. Fueldner, P. Gantenbein, P. Persdorf, X. Daguenet-Frick and F. Meunier, Faraday Discuss., 2021, 225, 384–402 RSC.
- H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. O. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424–428 CrossRef CAS PubMed.
- P. Falcaro, R. Ricco, C. M. Doherty, K. Liang, A. J. Hill and M. J. Styles, Chem. Soc. Rev., 2014, 43, 5513–5560 RSC.
- C. Serre, F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, D. Loueër and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519–13526 CrossRef CAS PubMed.
- P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, J. Am. Chem. Soc., 2008, 130, 6774–6780 CrossRef CAS PubMed.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle, M. Bosch and H.-C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- M. Eddaoudi, D. F. Sava, J. F. Eubank, K. Adila and V. Guillerm, Chem. Soc. Rev., 2015, 44, 228–249 RSC.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- R. Ricco, C. Pfeiffer, K. Sumida, C. J. Sumby, P. Falcaro, S. Furukawa, N. R. Champnessb and C. J. Doonan, CrystEngComm, 2016, 18, 6532–6542 RSC.
- R. Wen, J. Guo, A. Yu, J. Zhai and Z. L. Wang, Adv. Funct. Mater., 2019, 29, 1807655 CrossRef.
- G. Khandelwal, N. P. M. J. Raj and S.-J. Kim, Adv. Funct. Mater., 2020, 30, 1910162 CrossRef CAS.
- G. Khandelwal, A. Chandrasekhar, N. P. M. J. Raj and S.-J. Kim, Adv. Energy Mater., 2019, 9, 1803581 CrossRef.
- C. Huang, G. Lu, Y. Zhang, K. Zhu, S. Cui, W. Chen, Z. Wu, M. Qiu and L. Mi, Inorg. Chem., 2021, 60, 550–554 CrossRef CAS PubMed.
- Y.-Y. Zhang, M. Hu, Z. Shao, C. Huang, Q. Qin and L. Mi, CrystEngComm, 2021, 23, 5184–5189 RSC.
- Y. Zhang, J. Wu, S. Cui, W. Wei, W. Chen, R. Pang, Z. Wu and L. Mi, Chem. – Eur. J., 2020, 26, 584–591 CrossRef CAS PubMed.
- D. Li, W.-J. Shi and L. Hou, Inorg. Chem., 2005, 44, 3907–3913 CrossRef CAS PubMed.
- Z. Wang, L. Cheng, Y. B. Zheng, Y. Qin and Z. L. Wang, Nano Energy, 2014, 10, 37–43 CrossRef CAS.
- W. Guo, X. Li, M. Chen, L. Xu, L. Dong, X. Cao, W. Tang, J. Zhu, C. Lin, C. Pan and Z. L. Wang, Adv. Funct. Mater., 2014, 24, 6691–6699 CrossRef CAS.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- T. Gao, K. Zhao, X. Liu and Y. Yang, Nano Energy, 2017, 41, 210–216 CrossRef CAS.
- J. Zhang, Y. Zheng, L. Xu and D. Wang, Nano Energy, 2020, 69, 104435 CrossRef CAS.
- S. Cui, Y. Zheng, J. Liang and D. Wang, Chem. Sci., 2016, 7, 6477–6483 RSC.
- A. Maitra, S. Paria, S. K. Karan, R. Bera, A. Bera, A. K. Das, S. K. Si, L. Halder, A. De and B. B. Khatua, ACS Appl. Mater. Interfaces, 2019, 11, 5022–5036 CrossRef CAS.
- Z. Wang, Z. Ruan, W. S. Ng, H. Li, Z. Tang, Z. Liu, Y. Wang, H. Hu and C. Zhi, Small Methods, 2018, 2, 1800150 CrossRef.
- Y. Zi, S. Niu, J. Wang, Z. Wen, W. Tang and Z. L. Wang, Nat. Commun., 2015, 6, 8376–8384 CrossRef CAS PubMed.
- S. Cui, Y. Zheng, J. Liang and D. Wang, Nano Res., 2018, 11, 1873–1882 CrossRef CAS.
- M. Singh, A. Sheetal, H. Singh, R. S. Sawhney and J. Kaur, J. Electron. Mater., 2020, 49, 3409–3416 CrossRef CAS.
- X. Shi, S. Zhang and S. Gong, J. Mater. Chem., 2020, 8, 8997–9005 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, FT-IR spectra, dielectric frequency spectra and FE-SEM images. See DOI: 10.1039/d1ce01344k |
| This journal is © The Royal Society of Chemistry 2022 |