Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dual NHC/photoredox catalytic synthesis of 1,4-diketones using an MR-TADF photocatalyst (DiKTa)†
Callum
Prentice
ab,
James
Morrison
c,
Eli
Zysman-Colman
 *a and
Andrew D.
Smith
*a and
Andrew D.
Smith
 *b
*b
aOrganic Semiconductor Centre, EaStCHEM, School of Chemistry University of St Andrews, St Andrews, Fife, KY16, 9ST, UK. E-mail: eli.zysman-colman@st-andrews.ac.uk
bEaStCHEM, School of Chemistry, University of St Andrews, North Haugh, Fife, Scotland, KY16 9ST, UK. E-mail: ads10@st-andrews.ac.uk
cPharmaceutical Sciences, IMED Biotech Unit, Astra Zeneca, Macclesfield, SK10 2NA, UK
First published on 11th November 2022
Abstract
The use of the recently reported organic multi-resonant thermally activated delayed fluorescence (MR-TADF) photocatalyst DiKTa allows for the modular synthesis of 1,4-diketones under mild and metal-free conditions. The reaction proceeds via a three-component relay process in the presence of an N-heterocyclic carbene (NHC) organocatalyst.
The development of synthetic methodologies to produce 1,4-diketones is a well-studied area of research,1–3 in large part due to their utility as precursors of various heterocycles such as furans,4 thiophenes,5 and pyrroles.6 While a number of disconnections are possible, perhaps the most commonly used synthetic strategy follows the umpolung approach of disconnecting the 1,4-diketone into a nucleophilic acyl group equivalent and an α,β-unsaturated ketone, such as employed in the Stetter reaction.1 The Stetter reaction uses substoichiometric amounts of an N-heterocyclic carbene (NHC) in the presence of an aldehyde and an α,β-unsaturated ketone to furnish the desired 1,4-diketones via a nucleophilic Breslow intermediate. Another possible disconnection would involve generating the 1,4-diketone from three distinct fragments. While such processes have been developed employing conventional two electron mechanisms,7,8 they are currently limited to the use of 1,3-diketones in combination with an appropriate α-ketoaldehyde. As a more general approach, the 1,4-diketone may be disconnected into two acyl radical components and an alkene, which in the forward direction would involve a radical addition of one acyl group to the alkene, followed by a subsequent radical-radical coupling. This strategy has been executed successfully by the groups of Ackermann,9 Gilmour,10 Larionov,11 Li,12 and Wu.13 However, these examples are either limited to only symmetric 1,4-diketones or are restricted in terms of the scope of the acyl radical or alkenes available. The challenge of making unsymmetric 1,4-diketones using this strategy is significant, as the two acyl radicals must offer distinct reactivity to avoid forming a mixture of symmetric and unsymmetric products. Acyl radical generation and subsequent addition to alkenes using photoredox catalysis from commercially available, or simple to prepare, α-ketoacids, is well-known.10,13,14 Application to achieve selective formation of the desired unsymmetric 1,4-diketones requires another acyl radical, or equivalent, that must be generated simultaneously but itself will not react with the alkene. In a recent report Wang and Ackermann accomplished this transformation selectively through the use of metallaphotoredox catalysis using a tungsten polyoxometalate photocatalyst and a nickel co-catalyst.9 As an alternative, we envisaged that an acyl azolium intermediate, generated from an NHC catalyst and an appropriate electrophile, could undergo reduction through single electron transfer (SET) to the corresponding NHC-stabilised ketyl radical,15–17 which could act as the second acyl radical required for this transformation. During the course of our investigations Feng et al.18 published the use of triphenylphosphine in combination with NHC/photoredox dual catalysis using an iridium(III) photocatalyst for the generation of acyl radicals from benzoic acids generated in situ. However, to achieve the challenging synthesis of unsymmetric 1,4-diketones, the use of stoichiometric acyl azoliums was required (Scheme 1A). Building upon this work, we considered that using an α-keto acid in combination with the organic multi-resonant thermally activated delayed fluorescent (MR-TADF) photocatalyst DiKTa, recently reported by us,19 would allow for the preparation of unsymmetric 1,4-diketones in a modular, metal-free, three-component relay reaction using NHC/photoredox dual catalysis (Scheme 1B). During the preparation of this manuscript Zhang et al.20 published a similar study that relied upon the use of an Ir-based photocatalyst.
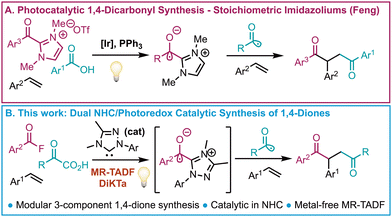 | ||
| Scheme 1 (A) Previous work using stoichiometric imidazolium species.18 (B) Present work using catalytic NHCs and DiKTa as the photocatalyst. | ||
Initial exploration used the reaction of commercially available benzoyl fluoride 1, styrene 2 and phenylglyoxylic acid 3 (Scheme 2 and ESI†). Using azolium salt precatalyst 4 in the presence of Cs2CO3 as a base and DiKTa, under photoexcitation using a 427 nm LED, the corresponding 1,4-dione 5 was isolated in good yield (entry 1). A range of alternative NHC catalysts such as 6 were tested but resulted in decreased product yield (entry 2 and ESI†). The use of Cs2CO3 as the base was found to be decisive, with substitution by alternative inorganic bases leading to diminished product yield (see ESI†). Various photocatalysts were also evaluated (see ESI†), with for example [Ir(ppy)2(dtbbpy)](PF6) giving 5 in a slightly reduced yield (entry 3). The use of alternative solvents such as toluene and acetonitrile were found to be mildly detrimental to product yield (entries 4–5 and ESI†). Control experiments verified the co-requirement of photocatalyst, NHC and light (entries 6–8). Observed side-products included a dimerization product of the initial radical addition intermediate, and an esterification product of benzoin (see ESI†).
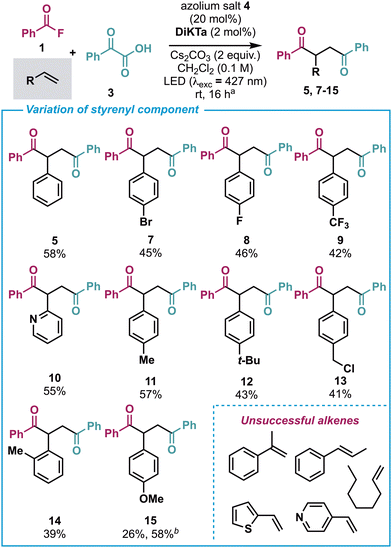
With the optimized conditions in hand (Scheme 2, entry 1), the scope and limitations of this NHC/photoredox dual catalysed synthesis of 1,4-diketones was developed. Using benzoyl fluoride 1 and phenylglyoxylic acid 3 a variety of terminal alkene derivatives was explored in this process (Scheme 3). Incorporation of halogenated (para-Br, para-F) as well as electron-withdrawing (para-CF3) substituents within the styrene component were tolerated, giving the desired 1,4-diketones in 42–46% yield (7–9). Incorporation of the heteroaromatic 2-vinyl pyridine variant produced comparable yield of product (10). The incorporation of alkyl substituents at the para position were well tolerated (11–13), while ortho substitution (to give 14) or electron-donating (para-MeO) substituents delivered the 1,4-diketone (15) in lower yields. However, 15 could be obtained in an improved yield when using an Ir-based photocatalyst and changing reaction stoichiometries.20 Substrates that proved unsuccessful under the developed conditions included α- and β-methyl styrene, 2-vinylthiophene, oct-1-ene and 4-vinylpyridine.
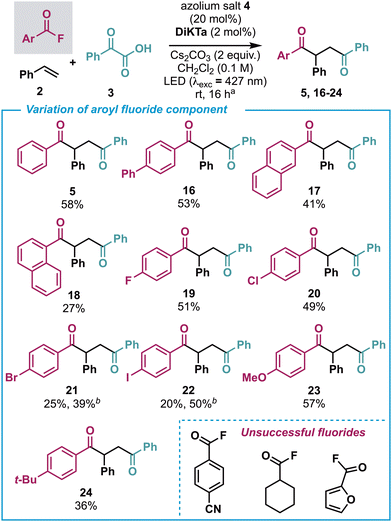
With the alkene scope established, attention turned to variation of the benzoyl fluoride component and the synthesis of unsymmetric 1,4-diketones (Scheme 4). In each case ∼5% of the symmetrical 1,4-diketone product was also observed, likely formed through the same process as reported by Wu and co-workers.13 Trace amounts of the corresponding 1,2-dione, where effectively the styrene component has been excluded, were also detected. Larger π-systems such as biphenyl (16) and 2-naphthyl (17) were well tolerated, although lower yields resulted from the use of 1-naphthyl (18), perhaps due to increased steric congestion. para-fluoro- (19) and para-chloro- (20) substituted benzoyl fluorides worked well; however, para-bromo (21) and para-iodo (22) derivatives gave reduced product yield. However, 21 and 22 could be obtained in improved yields using alternative conditions with the Ir-based photocatalyst.20 Pleasingly, electron-donating groups such as para-methoxy (23) and para-t-Bu (24) gave good yields of the corresponding unsymmetric 1,4-diketones. Unsuccessful substrates for the benzoyl fluoride component included para-cyanobenzoyl fluoride, cyclohexane carbonyl fluoride and 2-furanoyl fluoride.
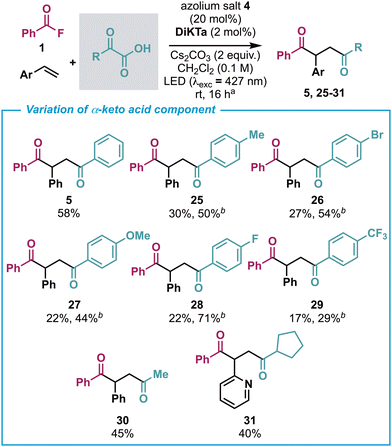
Given these promising results in terms of the breadth of both the alkene and benzoyl fluoride components, the scope of possible α-ketoacids was evaluated next (Scheme 5). Surprisingly, even seemingly small changes to the structure of the α-ketoacid such as the addition of a para-methyl substituent gave significantly lower yields of the corresponding 1,4-dione (25). This also proved to be the case for other substituents such as para-bromo, para-methoxy, para-fluoro and para-trifluoromethyl (26–29). However, using the alternative Ir photocatalyst-based catalytic conditions the yields for each of these substrates could be improved. Pleasingly, the use of pyruvic acid under standard conditions gave the corresponding 1,4-diketone (30) while the use of alkyl α-keto acids could be extended to the cyclopentyl variant with the use of 2-vinyl pyridine to give the corresponding 1,4-diketone (31).
A mechanism is proposed for the three-component transformation (Scheme 6A). Acylation of the in situ generated NHC by benzoyl fluoride [or by a bis(acyl) carbonate intermediate as proposed by Feng and co-workers18] leads to the acyl azolium ion pair intermediate I. Simultaneously the MR-TADF photocatalyst DiKTa (Ered(PC*/PC˙−) = 1.49 V vs. SCE) is generated in its excited state by absorption. This oxidizes the α-keto acid via SET (Eox([PhCOCO2][/K]) = 1.03 vs. SCE in DMSO),21 which undergoes decarboxylation to generates the key acyl radical intermediate. Addition of this acyl species to the least substituted terminus of the styrene generates the corresponding stabilised radical II. The reduced photocatalyst is oxidized via single electron reduction of I to generate NHC-stabilised ketyl intermediate III, closing the photocatalytic cycle. Subsequent radical-radical coupling of II and III give intermediate IV. Release of the NHC catalyst furnishes the desired 1,4-diketone product and completes the catalytic cycle. To support this mechanistic proposal, Stern–Volmer quenching studies of benzoyl fluoride 1, styrene 2 and phenylglyoxylic acid 3 were undertaken (Scheme 6B). These studies revealed that quenching occurs in the presence of 3 with a rate constant, kq, of 3.8 × 109 M−1s−1. Fluorescence quenching was not observed in the presence of 1 or 2. While these investigations support the plausibility of the proposed mechanism, they do not rule out the possibility of an alternative oxidative quenching mechanism.
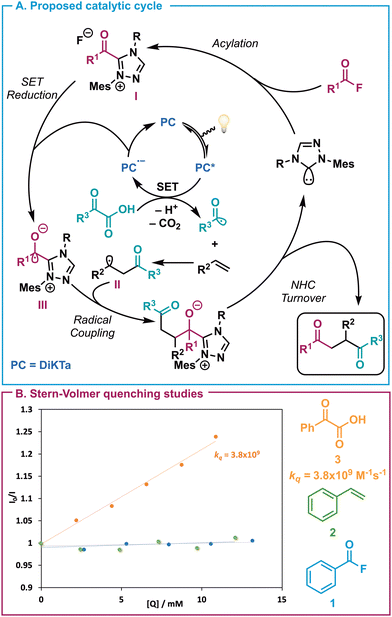 | ||
| Scheme 6 (A) Proposed mechanism of NHC/photoredox dual catalysed synthesis of 1,4-diones. (B) Stern–Volmer quenching studies. | ||
In summary, we have developed a modular synthetic route to unsymmetric 1,4-diketones through the combination of benzoyl fluorides, styrenes and α-keto acids catalysed by a dual catalytic NHC/photoredox system using the recently developed MR-TADF organophotocatalyst DiKTa.22 During the development of this system Zhang and co-workers published a similar methodology requiring an iridium-containing photocatalyst.20 Certain substrates performed better under their conditions, although most 1,4-diones were obtained in comparable yields using the metal-free conditions described herein. Further work will probe alternative strategies to utilise the reactivity of DiKTa in other photocatalytic transformations.
The authors thank AstraZeneca and the University of St Andrews for funding (Case studentship to C.P.).
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. M. Heravi, V. Zadsirjan, K. Kafshdarzadeh and Z. Amiri, Asian J. Org. Chem., 2020, 9, 1999–2034 CrossRef.
- W. H. García-Santos, J. B. Mateus-Ruiz and A. Cordero-Vargas, Org. Lett., 2019, 21, 4092–4096 CrossRef PubMed.
- W. Li, W. Wu, J. Yang, X. Liang and J. Ye, Synthesis, 2011, 1085–1091 Search PubMed.
- A. Deepthi, B. P. Babu and A. L. Balachandran, Org. Prep. Proced. Int., 2019, 51, 409–442 CrossRef.
- G. Minetto, L. F. Raveglia, A. Sega and M. Taddei, Eur. J. Org. Chem., 2005, 5277–5288 CrossRef.
- G. E. Veitch, K. L. Bridgwood, K. Rands-Trevor and S. V. Ley, Synlett, 2008, 2597–2600 Search PubMed.
- Q. Xia, X. Li, X. Fu, Y. Zhou, Y. Peng, J. Wang and G. Song, J. Org. Chem., 2021, 86, 9914–9923 CrossRef PubMed.
- J. Yang, F. Mei, S. Fu and Y. Gu, Green Chem., 2018, 20, 1367–1374 RSC.
- D. Wang and L. Ackermann, Chem. Sci., 2022, 13, 7256–7263 RSC.
- T. Morack, C. Mück-Lichtenfeld and R. Gilmour, Angew. Chem., Int. Ed., 2019, 58, 1208–1212 CrossRef CAS PubMed.
- S. Jin, X. Sui, G. C. Haug, V. D. Nguyen, H. T. Dang, H. D. Arman and O. V. Larionov, ACS Catal., 2022, 12, 285–294 CrossRef CAS.
- Q.-Z. Li, Y.-Q. Liu, X.-X. Kou, W.-L. Zou, P. Xiang, J.-D. Xing, T. Qi, X. Zhang and J.-L. Li, Angew. Chem., Int. Ed., 2022, 202207824 Search PubMed.
- Y. Cheng, J. Yu, T. Lei, H. Hou, B. Chen, C. Tung and L. Wu, Angew. Chem., 2021, 133, 27026–27032 CrossRef.
- M. Zhang, J. Xi, R. Ruzi, N. Li, Z. Wu, W. Li and C. Zhu, J. Org. Chem., 2017, 82, 9305–9311 CrossRef CAS.
- R. C. Betori, C. M. May and K. A. Scheidt, Angew. Chem., Int. Ed., 2019, 58, 16490–16494 CrossRef CAS PubMed.
- Q. Meng, N. Döben and A. Studer, Angew. Chem., Int. Ed., 2020, 59, 19956–19960 CrossRef CAS PubMed.
- L. Wang, R. Ma, J. Sun, G. Zheng and Q. Zhang, Chem. Sci., 2022, 13, 3169–3175 RSC.
- S. Li, H. Shu, S. Wang, W. Yang, F. Tang, X.-X. Li, S. Fan and Y.-S. Feng, Org. Lett., 2022, 24, 5710–5714 CrossRef CAS.
- D. Hall, S. M. Suresh, P. L. dos Santos, E. Duda, S. Bagnich, A. Pershin, D. B. Cordes, A. M. Z. Slawin, D. Beljonne, A. Köhler, I. D. W. Samuel, Y. Olivier and E. Zysman-Colman, Adv. Optical Mater., 2019, 1901627 Search PubMed; C. Prentice, J. Morrison, A. D. Smith and E. Zysman-Colman, Chem. – Eur. J., 2022 DOI:10.1002/chem.202202998.
- L. Wang, J. Sun, J. Xia, M. Li, L. Zhang, R. Ma, G. Zheng and Q. Zhang, Sci. China: Chem., 2022, 65, 1938–1944 CrossRef CAS.
- J. Liu, Q. Liu, H. Yi, C. Qin, R. Bai, X. Qi, Y. Lan and A. Lei, Angew. Chem., Int. Ed., 2014, 53, 502–506 CrossRef CAS PubMed.
- The research data supporting this publication can be accessed at: C. Prentice, J. Morrison, E. Zysman-Colman and A. D. Smith, 2022, data underpinning: Dual NHC/Photoredox Catalytic Synthesis of 1,4-Diketones Using an MR-TADF Photocatalyst (DiKTa). University of St Andrews Research Portal, DOI:10.17630/88844362-7585-4c97-905e-2ad1f580b68f.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc05705k |
| This journal is © The Royal Society of Chemistry 2022 |

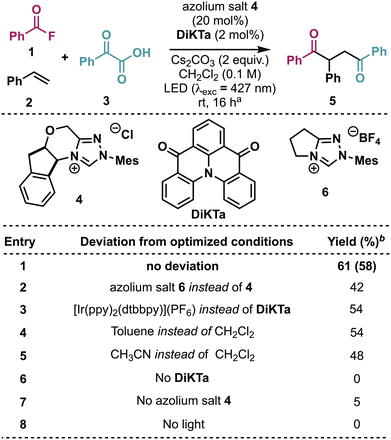
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Conditions: 1 (0.40 mmol), 2 (0.10 mmol), 3 (0.15 mmol), DiKTa (2 mol%), azolium salt 4 (20 mol%), CH2Cl2 (0.10 M), rt, N2, LED (
Conditions: 1 (0.40 mmol), 2 (0.10 mmol), 3 (0.15 mmol), DiKTa (2 mol%), azolium salt 4 (20 mol%), CH2Cl2 (0.10 M), rt, N2, LED (