Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
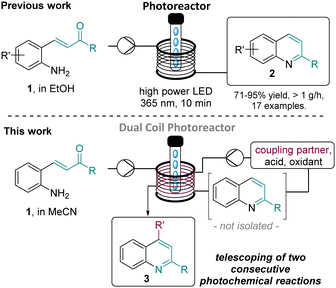
Consecutive photochemical reactions enabled by a dual flow reactor coil strategy†
Ruairi
Crawford‡
 a,
Mara
Di Filippo‡
a,
Mara
Di Filippo‡
 a,
Duncan
Guthrie
b and
Marcus
Baumann
a,
Duncan
Guthrie
b and
Marcus
Baumann
 *a
*a
aSchool of Chemistry, Science Centre South, University College Dublin, D04 N2E2, Dublin, Ireland. E-mail: marcus.baumann@ucd.ie
bVapourtec, Fornham St Genevieve, Bury St Edmunds, Suffolk, IP28 6TS, UK
First published on 8th November 2022
Abstract
The application of a dual reactor coil for consecutive photochemical reactions is presented in continuous flow mode. This strategy enables for the first time the use of a single LED-based light source to perform two distinct photochemical reactions in an uninterrupted fashion. This approach is demonstrated for the telescoped synthesis and functionalisation of drug-like quinolines and compared to alternatives exploiting two photochemical reactor set-ups operated in sequence. The presented strategy enables the intensified exploitation of photochemical reactions in modern synthesis.
The last decade has witnessed an extraordinary renaissance of synthetic photochemistry enabling the development of new methodology and its application for the generation of drug-like structures.1 The use of visible light in conjunction with photoredox catalysis2 thereby facilitates the selective functionalisation of advanced chemical entities, whereby UV irradiation remains crucial for the construction of desirable scaffolds including cyclobutanes that are not easily accessible by other means.1a,3 The concomitant evolution of continuous flow reactor technology and new light sources based on LEDs has additionally facilitated this development and at the same time enabled the uptake of this technology in many synthetic chemistry laboratories.4 Continuous flow photochemistry is seen by many as a powerful approach to not only enable the effective generation of target molecules exploiting light as an energy source and traceless reagent, but moreover to improve both the sustainability5 and scalability6 of these processes. Consequently, photochemistry enjoys growing popularity and is expected to have a bright future as its applications start to impact industrial processes.
Flow chemistry exploits the miniaturisation of reactors which provides for improved heat and mass transfer when compared to batch mode operation. Consequently, unstable and hazardous materials can be generated and consumed safely without handling of intermediates thus expanding the chemist's toolbox of accessible reactions.7 One additional benefit that is frequently exploited in flow-based reactions is the telescoping of individual transformations into an uninterrupted sequence.8 Reaction telescoping thereby streamlines the synthesis of target compounds leading to an overall increased efficacy of the process. Despite numerous accounts on successfully telescoped flow processes involving thermally driven reactions, telescoping for consecutive photochemical transformations remains unexplored despite its huge potential.9
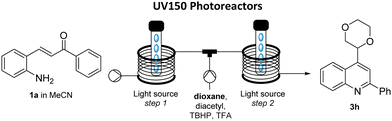
In this communication, we wish to demonstrate the use of a dual coil photoreactor for the effective telescoping of light-driven continuous processes. Specifically, this new strategy is demonstrated for the generation of quinolines and their telescoped functionalisation via diacetyl-catalysed Minisci reactions exploiting LEDs emitting in both the UV-A and visible range (i.e. 365 nm and 420 nm).
Our group recently reported on a photochemical method to generate a variety of substituted quinolines (2) from 2-aminochalcone precursors (1, Scheme 1, top).10 This method was developed in continuous flow mode and featured a high-power LED emitting at 365 nm that was found to give the targets in superior yields and purities when compared to using a classical medium-pressure Hg-lamp in combination with a filter.
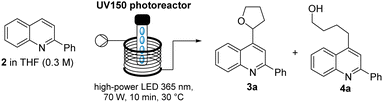
As this flow protocol tolerates the use of different solvents (e.g. MeCN, EtOH, acetone) and generates water as the only by-product, it was decided to directly build on this method to functionalise the quinoline core via a Minisci reaction.11 2-Phenylquinoline (2) was selected as model substrate and prepared in flow on multigram scale. Next, a survey for suitable conditions for Minisci reactions was performed establishing two attractive methods previously reported in batch mode. The first method was reported by Jin and co-workers12 and uses H2O2 as an oxidant in the presence of concentrated HCl. Various azacyclic scaffolds could be functionalised in good yields when irradiating the reaction mixture with a 427 nm LED as light source over a period of 18 h. An alternative method reported by Li and co-workers13 is based on diacetyl as photosensitiser in combination with TBHP and TFA and generates the desired products upon irradiation with a compact fluorescent lamp (CFL) over a period of 20–36 h.
Our studies commenced by evaluating both these methods for the continuous Minisci reaction of 2-phenylquinoline using THF as solvent and reaction partner. Directing a stock solution of 2 (0.3 M THF) in the presence of H2O2 (2 equiv.) through the UV flow reactor module (LED, 365 nm, 70 W input power) indicated full conversion of substrate within a short residence time of 10 minutes. However, in addition to the desired product (3a, 62%) a significant amount of the ring-opened congener 4a14 (31%, Table 1) was observed. Moreover, it was noticed that the use of a phase transfer catalyst would be necessary for less water-soluble ethers which made this approach less attractive to develop in flow mode. Therefore, our attention turned to the alternative method reported by Li and co-workers. Using analogous reaction conditions thereby rendered the desired target in a good yield and with excellent selectivity (0% of 4a).
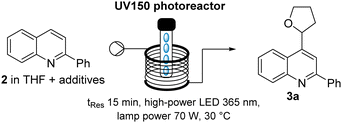
Based on these encouraging results, we decided to further explore the latter protocol for the desired continuous flow Minisci approach. Using a residence time of 15 minutes, the optimum concentration was found to be 0.3 M with lower and higher concentrations giving lower yields (Table 2, entries 1–3). At the same time, a reduction in residence time from 15 to 10 minutes did lead to a reduced yield (entry 4) as did reduction of the chosen input power (70 W to 50 W, entry 5). Lastly, reducing either the amount of diacetyl, TBHP or TFA did not improve the reaction performance (entries 6–8) indicating that the conditions of entry 2 would be most suitable going forward.
| Entry | Additive ratio: diacetyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBHP TBHP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TFA (in equiv.) TFA (in equiv.) |
THF (M) | Yield of 3aa (%) | Yield of 2a (%) |
|---|---|---|---|---|
| a 1H-NMR yield using trimethoxybenzene as internal standard. b t Res = 10 min. c Lamp power 50 W. | ||||
| 1 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.5 | 56 | 0 |
| 2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.3 | 67 | 5 |
| 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.1 | 63 | 8 |
| 4b | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.3 | 52 | 7 |
| 5c | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.3 | 57 | 2 |
| 6 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.3 | 46 | 13 |
| 7 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.3 | 54 | 0 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.3 | 54 | 12 |
Next, a small set of different quinolines and Minisci reaction partners were reacted under the best flow conditions (Scheme 2). This showed that the desired targets can be obtained in an accelerated fashion, although with varying yields (22–56%) due to small amounts of residual quinoline substrate in some cases. Importantly, this process showcased good tolerance of different aliphatic and aromatic moieties in the 2-position as well as a selection of ethers such as THF, 2-MeTHF, dioxane and Et2O as suitable Minisci reaction partners. Notably, these less commonly encountered ethers rendered the desired products in good yields and allowed to confirm the anticipated connectivity through single crystal diffraction in case of 3h.15
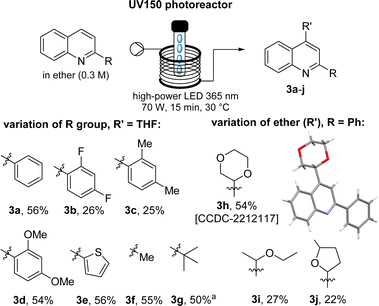 | ||
| Scheme 2 Substrate scope for continuous Minisci reaction (a 1H-NMR yield using trimethoxybenzene as internal standard). | ||
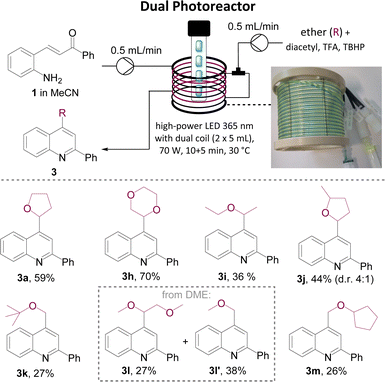
With a fast and robust protocol established for the flow based Minisci reaction we turned our attention to telescoping the two photochemical steps. A custom-built dual reactor coil consisting of two individual coils (5 mL, PTFE, see SI for details) with their respective entry and exit ports was utilised for this purpose. The previously described high-power LED emitting at 365 nm was exploited as it had facilitated both individual reactions as described above. The resulting set-up used a Vapourtec E-series flow reactor with its UV150 photo module (Scheme 3).
To start, a stock solution of the aminochalcone building block (1, 0.1 M, MeCN) was pumped through the first coil with a residence time of 10 minutes (0.5 mL min−1) which ensured full conversion of the substrate to the intermediate quinoline species (LED input power 70 W, ca. 30 °C reactor temperature). Upon exiting, this stream was mixed in a T-piece with a stock solution containing diacetyl (2 equiv.), TBHP (2 equiv.) and TFA (2 equiv.) dissolved in the desired ether (0.2 M). Evaluating this set-up to generate the dioxane-quinoline adduct 3h showed that a high yield of 70% can be obtained with a reduced residence time of 5 minutes for the Minisci reaction (total residence time: 15 min), which exceeded the results obtained when trialling the single-step Minisci protocol.16 Applying these improved conditions to a set of additional ethers gave the respective products in better yields and additionally facilitated the generation of Minisci products derived from methyl tertiary-butyl ether (MTBE), dimethyoxyethane (DME) and cyclopentyl methyl ether (CPME) in acceptable to good yields. Interestingly, the reaction with DME generated two products including 3l′ whereby fragmentation of a C–C bond was observed.17 Reaction with CPME generated a single regioisomer (3m) favouring reaction on the less substituted α-carbon in analogy to product 3j derived from 2-MeTHF.
Whilst the use of the dual photo reactor coil proved very effective in facilitating a telescoped photochemical process, it uses a single light source which may not be ideal for both photoreactions. To evaluate this scenario, we decided to study the telescoped photochemical synthesis of quinoline-based Minisci product 3h using two photoreactors equipped with different light sources (i.e. high-power LED at 365 nm, 70 W input power and medium-power LED at 425 nm, 18 W radiant power).
As shown in Table 3 using a blue LED as light source for the Minisci reaction in combination with the high-power LED emitting at 365 nm does not give full conversion of the quinoline intermediate 2 to the dioxane adduct (3h, entry 1). Conversely, using the blue LED for step 1 and the high-power LED for step 2 generates the desired product 3h in a high yield of 70% (entry 2) equivalent to using the high-power LED for both steps (entry 3). Lastly, using the blue LED for both steps generates product 3h in 34% yield along with significant amounts of the quinoline intermediate (2, 54%, entry 4). These data clearly show that the blue LED is tolerated in the quinoline forming step whereas the Minisci reaction performs much better with the high-power LED emitting at 365 nm. Therefore, the most effective set-up for performing this telescoped photo reaction sequence comprises of the newly developed dual coil photo reactor in conjunction with the high-power LED as single light source (365 nm) for both steps.
| Entry | Step 1 tRes = 10 min | Step 2 tRes = 5 min | Yield of 3ha |
|---|---|---|---|
| a 1H-NMR yield using trimethoxybenzene as internal standard. | |||
| Two-photoreactor set-up | |||
| 1 | High-power LED, 365 nm | Blue LED, 425 nm | 23% (2: 62%) |
| 2 | Blue LED, 425 nm | High-power LED, 365 nm | 70% |
| Dual coil reactor set-up (Scheme 3) | |||
| 3 | High-power LED, 365 nm | High-power LED, 365 nm | 70% |
| 4 | Blue LED, 425 nm | Blue LED, 425 nm | 34% (2: 54%) |
In conclusion, we have developed a new dual coil photoreactor set-up to perform consecutive photochemical reactions in flow mode. This set-up is modular and readily combined with commercial flow reactor technology and provides for easy scalability. Our study showcases that a photochemical quinoline synthesis can be telescoped with a photochemical Minisci reaction to produce small sets of drug-like quinolines functionalised with different ethers. Crucially, this flow approach allows for process intensification as the target compounds can be accessed in a combined residence time of 15 minutes. It is demonstrated that the dual coil set-up is more effective than an analogous set-up comprising of two flow reactors and two LED-based light sources. Given the high demand for modern photochemical transformations for both the synthesis and functionalisation of drug-like scaffolds, we believe that our approach for reaction telescoping for distinct photochemical reactions will inspire more studies in this important field.
We are grateful to the School of Chemistry for providing a Sir Walter Hartley Scholarship (to MDF). SFI is acknowledged for support through the 2018 Infrastructure Call (18/RI/5702) and the European Development Fund (12/RC2275_P2). We thank Dr Andrew D. Philips for solving the crystal structure reported in this communication. MB thanks the Royal Society of Chemistry for a Research Enablement Grant (E20-2998).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) T. Bach and J. P. Hehn, Angew. Chem., Int. Ed., 2011, 50, 1000 CrossRef CAS PubMed; (b) M. D. Kärkäs, J. A. Porco Jr. and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683 CrossRef PubMed; (c) H. E. Zimmerman, Pure Appl. Chem., 2006, 78, 2193 CrossRef CAS; (d) N. Hoffmann, Chem. Rev., 2008, 108, 1052 CrossRef CAS PubMed.
- (a) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS PubMed; (b) J. Liu, L. Lu, D. Wood and S. Lin, ACS Cent. Sci., 2020, 6, 1317 CrossRef CAS PubMed; (c) M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898 CrossRef CAS PubMed.
- S. Poplata, A. Troester, Y.-Q. Zou and T. Bach, Chem. Rev., 2016, 116, 9748 CrossRef CAS PubMed.
- (a) T. H. Rehm, Chem. – Eur. J., 2020, 26, 16952 CrossRef CAS PubMed; (b) L. Buglioni, F. Raymenants, A. Slattery, S. T. A. Zondag and T. Noël, Chem. Rev., 2022, 122, 2752 CrossRef CAS PubMed; (c) M. Di Filippo, C. Bracken and M. Baumann, Molecules, 2020, 25, 356 CrossRef CAS PubMed.
- (a) J. D. Williams and C. O. Kappe, Curr. Opin. Green Sustainable Chem., 2020, 25, 100351 CrossRef; (b) L. Rogers and K. F. Jensen, Green Chem., 2019, 21, 3481 RSC; (c) A. I. Alfano, M. Brindisi and H. Lange, Green Chem., 2021, 23, 2233 RSC; (d) M. Baumann, T. S. Moody, M. Smyth and S. Wharry, Synthesis, 2021, 3963 CrossRef CAS.
- (a) K. Donnelly and M. Baumann, J. Flow Chem., 2021, 11, 223 CrossRef CAS; (b) E. Kayahan, M. Jacobs, L. Braeken, L. C. J. Thomassen, S. Kuhn, T. van Gerven and M. E. Leblebici, Beilstein J. Org. Chem., 2020, 16, 2484 CrossRef CAS PubMed.
- (a) D. Dallinger, B. Gutmann and C. O. Kappe, Acc. Chem. Res., 2020, 53, 1330 CrossRef CAS PubMed; (b) T. Razzaq and C. O. Kappe, Chem. – Asian J., 2010, 5, 1274 CAS; (c) A. Bonner, A. Loftus, A. Padgham and M. Baumann, Org. Biomol. Chem., 2021, 86, 14199 CrossRef PubMed; (d) M. Movsisyan, E. I. P. Delbeke, J. K. E. T. Berton, C. Battilocchio, S. V. Ley and C. V. Stevens, Chem. Soc. Rev., 2016, 45, 4892 RSC; (e) B. J. Deadman, S. G. Collins and A. R. Maguire, Chem. – Eur. J., 2015, 21, 2298 CrossRef CAS PubMed.
- (a) J. Britton and C. L. Raston, Chem. Soc. Rev., 2017, 46, 1250 RSC; (b) M. A. Morin, W. Zhang, D. Mallik and M. G. Organ, Angew. Chem., Int. Ed., 2021, 60, 20606 CrossRef CAS PubMed; (c) J. Jiao, W. Nie, T. Yu, F. Yang, Q. Zhang, F. Aihemaiti, T. Yang, X. Liu, J. Wang and P. Li, Chem. – Eur. J., 2021, 27, 4817 CrossRef CAS PubMed.
- In an earlier report by the Collins group, an example is provided where two consecutive photochemical flow reactions were performed using different light sources. For details, please see: S. Parisien-Collette and S. Collins, ChemPhotoChem, 2018, 2, 855 CrossRef CAS.
- M. Di Filippo and M. Baumann, Eur. J. Org. Chem., 2020, 6199 CrossRef CAS.
- R. S. J. Proctor and R. J. Phipps, Angew. Chem., Int. Ed., 2019, 58, 13666 CrossRef CAS PubMed.
- H. Zhao, Z. Li and J. Jin, New J. Chem., 2019, 43, 12533 RSC.
- C.-Y. Huang, J. Li, W. Liu and C.-J. Li, Chem. Sci., 2019, 10, 5018 RSC.
- In other reports, the formation of 4a by ring opening is described as the only product under photochemical conditions, e.g.: C. Y. Huang, J. Li and C. J. Li, Nat. Commun., 2021, 12, 4010 CrossRef CAS PubMed.
- The crystal data for compound 3h has been deposited with the Cambridge Crystallographic Data Centre as CCDC 2212117†.
- The increased yield may arise from using a solvent mixture containing MeCN and the ethereal solvent.
- Fragmentation of DME-derived Minisci products has been reported in ref. 14, although cleavage of the C–O bond was observed instead of the C–C bond.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and data, and copies of NMR spectra. CCDC 2212117. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc05601a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |