 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
More than ADEQUATE: doubling the sensitivity of 13CH–13CH correlations in double-quantum NMR experiments†
Justinas
Sakas
 and
Dušan
Uhrín
and
Dušan
Uhrín
 *
*
EaStCHEM School of Chemistry, University of Edinburgh, Edinburgh, UK. E-mail: dusan.uhrin@ed.ac.uk
First published on 31st October 2022
Abstract
We present modifications of the ADEQUATE experiment which more than double the sensitivity of carbon–carbon correlations of 13CH–13CH moieties. Additionally, these improvements can be applied without a sensitivity penalty to obtain spectra with a 13C chemical shift axis in the indirectly detected dimension, instead of a double-quantum frequency, allowing simpler interpretation of spectra. The modified experiments, which use refocussing of 1JCH couplings and 1H decoupling during JCC evolution intervals, were tested on several molecules, including a pentasaccharide (20 mg, 19 mM), where on average a 2.6-fold signal-to-noise improvement was achieved and the number of observable correlations increased. Doubling sensitivity results in a 4-fold reduction of the experimental time, allowing ADEQUATE spectra to be recorded overnight instead of over multiple days.
The INADEQUATE (incredible natural abundance double-quantum transfer experiment) NMR experiment1,2 is of substantial interest to chemists as it allows tracing out the carbon skeleton of a molecule.3 It relies on the detection of double-quantum (DQ) coherences between two coupled 13C spins and therefore has inherently inadequate sensitivity, as the probability of a molecule containing a pair of 13C atoms is approximately 1-in-8300. Historically, this has limited its use, as long experimental times and/or high sample concentrations were typically required. However, the development of cryogenically cooled NMR probes and the associated sensitivity gains enabled INADEQUATE and its variants to become routine experiments, which have been reviewed extensively.4–9
For protonated 13C atoms, sensitivity of INADEQUATE can be increased by using 1H detection as demonstrated by the INEPT-INADEQUATE experiment.10 The addition of the INEPT (insensitive nuclei enhancement by polarisation transfer) step offers increased sensitivity due to the higher gyromagnetic ratio (γ) of 1H compared to 13C. Considering proton singlets and neglecting the sensitivity enhancement of 13C-detected INADEQUATE through heteronuclear NOE, the maximum theoretical signal-to-noise ratio (SNR) increase associated with 1H detection is (γH/γC)5/2 ≈ 32.11 However, such a SNR increase is not achieved for various reasons.12 A factor of √2 is lost due to pulsed-field gradient (PFG) selection,13 and a loss of a further factor of 2 occurs because in the 1H-detected experiment the DQ coherences start and end on the same proton, whereas in 13C-detected INADEQUATE the coherences are generated from two nuclei. Additional sensitivity loses occur due to relaxation effects: the INEPT transfer of the 1H-detected experiments generates mixed proton–carbon coherences that in medium size molecules relax faster than pure carbon coherences. While sharp 1H-decoupled antiphase 13C–13C doublets are acquired in 13C-detected INADEQUATE, complex 1H multiplets degrade sensitivity of 1H-detected methods.
As a result, approaches have been developed to improve the sensitivity of 1H detection. It has been demonstrated that removing the proton term from mixed CH coherences prolongs their relaxation times and increases the overall sensitivity of experiments.12 This approach is especially beneficial for long-range (nJCC, n ≥ 2) optimised 1H-detected experiments containing longer nJCC evolution delays. The sensitivity of 1H-detected INADEQUATE was substantially increased by 1H-detected ADEQUATE (adequate sensitivity double-quantum spectroscopy).14,15 This method employs a 120° 13C pulse to optimise the 13C multiple-quantum to single-quantum coherence transfer and incorporates the preservation of equivalent pathways (PEP) element that offers sensitivity improvements of up to √2-fold.16 Sensitivity can be further improved by homonuclear decoupling that at least partially simplifies the structure of 1H multiplets.17,18
In order to avoid complications associated with the setup and interpretation of experiments that produce DQ frequencies in the indirectly detected dimension (F1), a version of the ADEQUATE experiment was reported that samples 13C single-quantum (SQ) coherences in F1.14,15 This modification, originally termed ω1-refocussed ADEQUATE, is referred to as SQ ADEQUATE herein. It works by incorporating an additional t1 period into the pulse sequence, during which the 13C–13C DQ coherences are modulated by a single-quantum 13C frequency with an opposite sign to the DQ 13C–13C frequency. For an Hn-Cn-Cm-Hm spin system this amounts to Hn being modulated by the Cm frequency (and Hm by Cn), and thus the 1JCC-optimised SQ ADEQUATE experiment produces a spectrum containing pseudo-2-bond C–H correlations at the (F2, F1) chemical shifts of (Hn, Cm) and (Hm, Cn). A comparison of SQ ADEQUATE and 2D 1H, 13C HSQC spectra (the latter acquired in a fraction of time) allows for a straightforward interpretation of 13C–13C correlations observed in SQ ADEQUATE experiments.
In this work, we present modifications of the ADEQUATE experiments that significantly increase the sensitivity of 13C–13C correlations for molecular moieties containing CH–CH fragments. These modifications can be applied to experiments that sample either DQ or SQ frequencies in F1 and are herein referred to collectively as 1JCH-refocussed ADEQUATE (Fig. 1). The full pulse sequence parameters and experimental details are presented in Fig. S1 (ESI†).
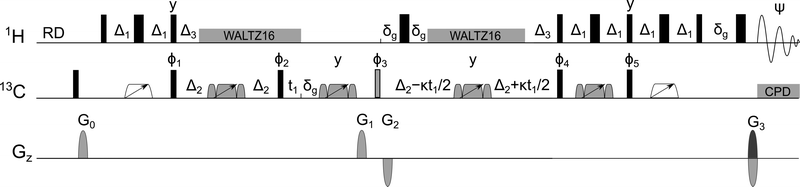 | ||
| Fig. 1 1 J CH-refocussed ADEQUATE pulse sequence. If κ = 0, double-quantum chemical shift is obtained in F1. If κ = 1, single-quantum 13C chemical shift is obtained in F1. For full description of parameters, see ESI.† | ||
The first minor modification of the original experiments is the addition of a 90° 13C pulse and a PFG purge element at the beginning of the pulse sequence, which defocusses any 13C magnetisation, thus eliminating polarisation transfer pathways starting on 13C. Although the ADEQUATE experiment employs phase cycling and PFG selection to remove the strong signals coming from 12C-bonded protons, isolated 13C-bonded protons or 13C atoms, imperfect defocussing of these signals can obscure correlations of interest. The addition of this purge element reduced cancellation artefacts and slightly increased the SNR as shown in Fig. S2 (ESI†).
A major sensitivity increase was achieved by refocussing the 1JCH coupling at the beginning of the 1/(21,nJCC) evolution interval following the initial 1H → 13C INEPT transfer. This conversion of carbon-proton antiphase coherences into pure 13C coherences prior to excitation of DQ coherence removes the leakage of signal into undetectable zero-quantum coherences and generates a 2-fold sensitivity increase.19 Application of 1H decoupling while SQ or DQ 13C coherences are present, reduces relaxation and further increases the sensitivity of experiments.12 To regenerate the antiphase proton–carbon coherences required for the reverse 13C → 1H INEPT step, the decoupling is turned off for a period of 1/(21JCH) at the end of the 1/(21,nJCC) refocussing interval.
The increased SNR of the modified ADEQUATE experiments was evaluated using a concentrated sample of methyl β-D-xylopyranoside (I, 1.1 M, Scheme 1). This model compound was chosen because it contains CH, CH2 and CH3 carbons; its high concentration enabled fast and accurate comparison of related spectra. Relative SNR changes for CH groups are presented in Table 1 and Fig. 2; full spectra are shown in Fig. S3 (ESI†). SNR improvement for selected correlations is highlighted in Fig. S4 (ESI†). Not reported previously in the literature, these results indicated that the original 1JCC-optimised SQ (ω1-refocussed) ADEQUATE experiment shows an up to 32% sensitivity loss compared to the DQ ADEQUATE experiment. This loss, which can be tolerated in exchange for a more straightforward analysis of spectra when sample concentration is not limiting, is associated with the appearance of HSQC-like artefacts at frequencies of (Hn, Cn) as shown in Fig. S3c (ESI†). These extra correlations, which can be much more efficiently obtained from 1H, 13C HSQC spectra, can hinder analysis of complicated spectra by introducing peak overlap. The proposed 1JCH-refocussed SQ ADEQUATE eliminates this leakage (cf. Fig. S3c and d, ESI†), which explains larger SNR increases observed for the 1JCC-optimised SQ compared to the DQ experiment (2.1× vs 1.7×). Long-range SQ ADEQUATE spectra do not show significant HSQC-like artefacts, hence the increase in sensitivity is comparable for the DQ and SQ experiment (2.3× for both).
 | ||
| Scheme 1 Compounds used for ADEQUATE experiments: methyl β-D-xylopyranoside (I), strychnine (II) and fondaparinux (III). | ||
| Experiment | SNR fold change | |
|---|---|---|
| One-bonda | Long-rangeb | |
| a n (number of correlations) = 6. b n = 4. c With respect to original DQ ADEQUATE. d With respect to original SQ ADEQUATE. | ||
| DQ ADEQUATE | 1.0 | 1.0 |
| SQ ADEQUATEc | 0.9 ± 0.1 | 0.9 ± 0.1 |
| 1 J CH-refocussed DQc | 1.7 ± 0.3 | 2.3 ± 0.3 |
| 1 J CH-refocussed SQc | 1.8 ± 0.3 | 2.0 ± 0.4 |
| 1 J CH-refocussed SQd | 2.1 ± 0.2 | 2.3 ± 0.4 |
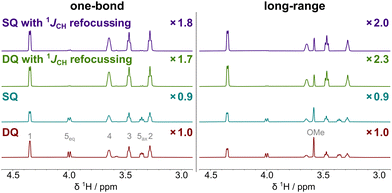 | ||
| Fig. 2 Positive projections of ADEQUATE spectra of I optimised for one-bond (45 Hz) and long-range (6 Hz) JCC couplings. The factors indicate the average SNR improvement for CH groups compared to DQ ADEQUATE. Full one-bond 2D spectra are shown in Fig. S3 (ESI†). | ||
Overall, the data summarised in Table 1 indicate that 1JCH-refocussed SQ ADEQUATE experiment performs best for both one-bond and long-range 13CH–13CH correlations. Therefore, simpler spectra, displaying SQ 13C chemical shift along F1 can now be obtained without a sensitivity penalty and with a 1.8–2.0-fold enhancement compared to original DQ ADEQUATE. As the 1JCH-refocussed ADEQUATE experiments are designed to refocus couplings of CH groups only, the sensitivity of CH3-detected correlations is reduced and CH2-detected correlations are absent. The CH-detected CH–CHx (x = 0, 2, 3) connectivities are present with improved sensitivity (1.3–2.0×) compared to the original ADEQUATE spectra as evaluated on the spectra of L-isoleucine (Fig. S5, ESI†).
In order to investigate the SNR improvements on a compound with a more diverse structure, one-bond and long-range optimised SQ ADEQUATE spectra were acquired for strychnine (II, 32 mM). Despite the wide range of coupling constants reported for II (1JCH = 124–168 Hz, 1JCC = 32–71 Hz, 3JCC = 3–7 Hz),20,21 experiments optimised for 1JCH (150 Hz) and 1,nJcc (50 Hz or 6 Hz) yielded sensitivity improvements similar to those observed for I. A SNR gain of (1.9 ± 0.4)× was observed for CH–CH correlations in 1JCC-optimised ADEQUATE (Fig. S6 and Table S1, ESI†). A more significant increase in sensitivity was observed in the long-range spectra (Fig. S7 and Table S2, ESI†), with an average SNR increase of 2.3× for CH–CH correlations (Fig. 3). The sensitivity is also improved for CH–CHx (x = 0, 2) moieties, although by not as much, especially for quaternary carbons. The lower increase can be attributed to quaternary carbons of II having relaxation times of ≥20 s,22 therefore not benefiting much from reducing relaxation losses.
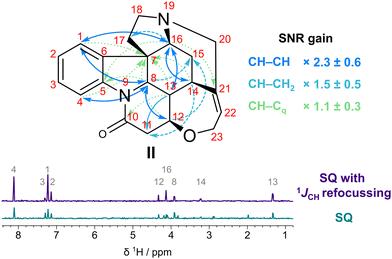 | ||
| Fig. 3 Correlations observed and the SNR improvement achieved by 1JCH refocussing in nJCC-optimised SQ ADEQUATE experiments for II. F2 projection with and without 1JCH refocussing are shown. Full spectra are presented in Fig. S7 (ESI†). Note that correlations observed for the aromatic system have been omitted for clarity. | ||
To illustrate the benefits of the improved ADEQUATE experiment on a weaker sample of a larger molecule, one-bond and long-range 13CH–13CH correlations of a sulfated pentasaccharide, fondaparinux Na (III, Scheme 1, Mw = 1728 g mol−1, 19 mM), were obtained on an 800 MHz spectrometer equipped with a TCI cryoprobe in 7 and 21 hours, respectively (Fig. 4 and Fig. S8–S10, ESI†). SNR improvements in the range of 1.4- to 4.4-fold were obtained for the 1JCH-refocussed SQ ADEQUATE experiment optimised for 1JCC coupling constants (Table S3, ESI†). Low SNR of the spectra is responsible for such large variations, yielding an average (2.6 ± 0.7)-fold sensitivity increase. Increased SNRs were also seen in the nJCC-optimised 1JCH-refocussed SQ ADEQUATE spectra (2.2 ± 0.5-fold, Table S4, ESI†). The increase in sensitivity is larger than for I or II, which illustrates that larger molecules benefit more from reduced relaxation losses due to their shorter 1H relaxation times.
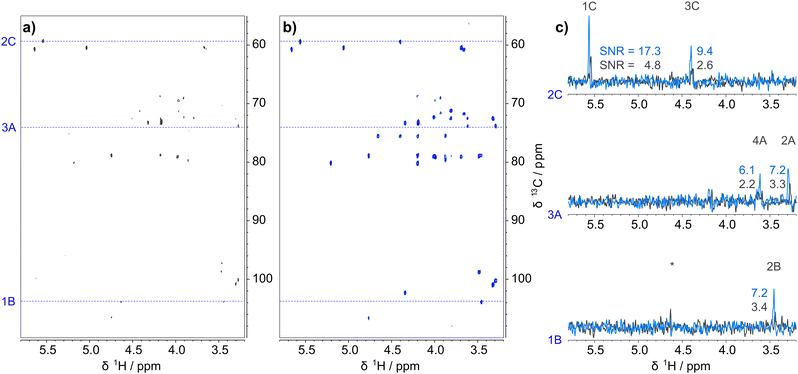 | ||
| Fig. 4 1 J CC-optimised SQ ADEQUATE spectra of III (a) without (black) and (b) with 1JCH refocussing (blue). The spectra were recorded for the same length of time and are plotted at the same intensity level. Comparison of marked traces (c) shows the sensitivity gains obtained by refocussing. Note that the 1B trace for spectrum (a) contains a weak HSQC-like artefact (*) which could be confused with a genuine correlation. For full peak assignment, see Fig. S8 (ESI†). | ||
The sensitivity increase in both one-bond and long-range optimised 1JCH-refocussed experiments is significant, however the values only refer to SNR increases for signals present in both the original and improved spectra. In fact, 1JCH-refocussing revealed extra correlations not present in the spectra of the original experiments, therefore the sensitivity gains are even larger than the calculated factors. This is particularly true for the nJCC-optimised experiments.
Correlations between adjacent monomer units in the pentasaccharide, providing valuable ring connectivity information, were observed. These experiments demonstrate that with <4 mg per monosaccharide residue, 1JCH-refocussed SQ ADEQUATE is an efficient experiment for structure determination of oligosaccharides.
It is worth noting that due to fast evolution of 1JCC compared to nJCC coupling constants, one-bond correlations can appear in long-range optimised spectra. In this work, these correlations were identified by comparison to 1JCC-optimised spectra, however methods exist to allow discrimination between 1JCC and nJCC correlations in nJCC-optimised experiments.23
In conclusion, the pulse sequences presented in this work provide significant improvements for the detection of 13CH–13CH correlation by double-quantum NMR experiments. Larger than 2-fold increases have been achieved, which translate to a 4-times reduction in spectrometer time. Double quantum 13C–13C experiments are regularly recorded over multiple days, yet the sensitivity-improved experiments will be able to provide the same information overnight. The new method is compatible with other schemes, such as homonuclear decoupling and non-uniform sampling, that have already been successfully applied to ADEQUATE experiments.17,18,24,25 Additionally, the proposed modifications allow observation of SQ 13C frequency in the F1 dimension without a sensitivity penalty. These sensitivity enhancements increase the potential of using 13C–13C correlations for structure elucidation and will benefit areas such as carbohydrates, natural products or mixture analysis, as well as general NMR applications when sample quantities are limited.
This research was supported by EPSRC grants EP/T517884/1 and EP/R030065/1. The authors thank Mr Juraj Bella and Dr Lorna Murray for the maintenance of the NMR spectrometer. Raw data for the spectra presented in this communication can be found at https://doi.org/10.7488/ds/3779.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Bax, R. Freeman and S. P. Kempsell, J. Am. Chem. Soc., 1980, 102, 4849–4851 CrossRef CAS.
- A. Bax, R. Freeman and T. A. Frenkiel, J. Am. Chem. Soc., 1981, 103, 2102–2104 CrossRef CAS.
- J. Buddrus and H. Bauer, Angew. Chem., Int. Ed. Engl., 1987, 26, 625–642 CrossRef.
- J. Buddrus and J. Lambert, Magn. Reson. Chem., 2002, 40, 3–23 CrossRef CAS.
- E. Kupče and R. Freeman, J. Am. Chem. Soc., 2008, 130, 10788–10792 CrossRef PubMed.
- D. Uhrín, in Annual Reports on NMR Spectroscopy, ed. G. Webb, Academic Press, 2010, vol. 70, pp. 1–34 Search PubMed.
- G. E. Martin, in Annual Reports on NMR Spectroscopy, ed. G. A. Webb, Academic Press, 2011, vol. 74, pp. 215–291 Search PubMed.
- G. E. Martin, M. Reibarkh, A. V. Buevich, K. A. Blinov and R. T. Williamson, eMagRes, 2014, 3, 215–234 CAS.
- J. Saurí, I. E. Ndukwe, M. Reibarkh, Y. Liu, R. T. Williamson and G. E. Martin, in Annual Reports on NMR Spectroscopy, ed. G. A. Webb, Academic Press, 2019, vol. 98, pp. 1–56 Search PubMed.
- J. Weigelt and G. Otting, J. Magn. Reson., Ser. A, 1995, 113, 128–130 CrossRef CAS.
- P. K. Mandal and A. Majumdar, Concepts Magn. Reson., 2004, 20A, 1–23 CrossRef CAS.
- L. Jin, K. E. Kövér, M. R. Lenoir and D. Uhrín, J. Magn. Reson., 2008, 190, 171–182 CrossRef CAS PubMed.
- A. Ross, M. Czisch, C. Cieslar and T. Holak, J. Biomol. NMR, 1993, 3, 215–224 CrossRef CAS.
- M. Köck, B. Reif, W. Fenical and C. Griesinger, Tetrahedron Lett., 1996, 37, 363–366 CrossRef.
- B. Reif, M. Köck, R. Kerssebaum, H. Kang, W. Fenical and C. Griesinger, J. Magn. Reson., Ser. A, 1996, 118, 282–285 CrossRef CAS.
- J. Cavanagh and M. Rance, in Annual Reports on NMR Spectroscopy, ed. G. A. Webb, Academic Press, 1993, vol. 27, pp. 1–58 Search PubMed.
- J. Saurí, W. Bermel, A. V. Buevich, E. C. Sherer, L. A. Joyce, M. H. M. Sharaf, P. L. Schiff, T. Parella, R. T. Williamson and G. E. Martin, Angew. Chem., Int. Ed., 2015, 54, 10160–10164 CrossRef PubMed.
- J. Saurí, W. Bermel, T. Parella, R. Thomas Williamson and G. E. Martin, Magn. Reson. Chem., 2018, 56, 1029–1036 CrossRef PubMed.
- A. Meissner, D. Moskau, N. C. Nielsen and O. W. Sørensen, J. Magn. Reson., 1997, 124, 245–249 CrossRef CAS.
- A. V. Buevich, J. Saurí, T. Parella, N. De Tommasi, G. Bifulco, R. T. Williamson and G. E. Martin, Chem. Commun., 2019, 55, 5781–5784 RSC.
- R. T. Williamson, A. V. Buevich and G. E. Martin, Org. Lett., 2012, 14, 5098–5101 CrossRef CAS PubMed.
- H. Fujiwara, Y.-Z. Da, T. Takagi and Y. Sasaki, Chem. Pharm. Bull., 1989, 37, 2887–2891 CrossRef CAS.
- G. E. Martin, R. T. Williamson, P. G. Dormer and W. Bermel, Magn. Reson. Chem., 2012, 50, 563–568 CrossRef CAS PubMed.
- J. Saurí, T. Parella, R. T. Williamson and G. E. Martin, Magn. Reson. Chem., 2017, 55, 191–197 CrossRef PubMed.
- M. S. Roginkin, I. E. Ndukwe, D. L. Craft, R. T. Williamson, M. Reibarkh, G. E. Martin and D. Rovnyak, Magn. Reson. Chem., 2020, 58, 625–640 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, additional results, NMR spectra and pulse programs. See DOI: https://doi.org/10.1039/d2cc05214h |
| This journal is © The Royal Society of Chemistry 2022 |
