Open Access Article
Open Access ArticleGauging the donor strength of iron(0) complexes via their N-heterocyclic carbene gold(I) adducts†
Zhi Hao
Toh
a,
Hendrik
Tinnermann
a,
Dinh Cao Huan
Do
 a,
Han Vinh
Huynh
a,
Han Vinh
Huynh
 a,
Tobias
Krämer
a,
Tobias
Krämer
 b and
Rowan D.
Young
b and
Rowan D.
Young
 *a
*a
aDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, 117543, Singapore. E-mail: rowan.young@nus.edu.sg
bDepartment of Chemistry, Maynooth University, Maynooth, Co. Kildare, Ireland
First published on 1st November 2022
Abstract
We isolate and characterize the gold(I)–iron(0) adducts [(iPr2-bimy)Au–Fe(CO)3(PMe3)2][BArF4] and [Au–{Fe(CO)3(PMe3)2}2][BArF4] (iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene, BArF4 = tetrakis(pentafluorophenyl)borate). DFT analysis reveals that the gold–iron interaction in [(iPr2-bimy)Au–Fe(CO)3(PMe3)2][BArF4] is predominantly a σ-donation from iron to gold. We further extend this class of compounds to include [(iPr2-bimy)Au–Fe(CO)3(PR3)2][BArF4] (PR3 = PPh3, PCy3, PCyPh2, PMePh2, PMe2Ph, P(4-C6H4F)3) and [(iPr2-bimy)Au–Fe(CO)4(PPh3)][BArF4] and correlate the iPr2-bimy carbenic 13C NMR signal with the relative donor strength of the iron(0) ligand. This approach allows for a fast and simple approach to gauge relative donor strength of Fe(0) donors.
Transition metals hold a traditional role as Lewis acids in activation and coordination chemistries. However, the use of metals as bases in metal-only Lewis pair (MOLP) and frustrated Lewis pair (FLP) systems has recently become well recognized (Fig. 1).1 Metal base partners in FLP and MOLP systems result in enhanced activity and a plethora of post-activation functionalization options that are unavailable to main-group Lewis bases. Notably, this allows the use of first row transition metals in difficult catalytic transformations that commonly rely upon noble metals.2
 | ||
| Fig. 1 (i) Zero valent iron complexes used as base partners in FLP chemistry.3 (ii) Zero valent iron complexes acting as ligands.5 (iii) Correlation between the carbenic 13C NMR signal of iPr2-bimy in [(iPr2-bimy)AuL]+ and donor strength was shown for L = NHC providing a fast and accurate way to quantify the donor strength of ligands via NMR spectroscopy.9 Can it be applied in an analogous manner to transition metals such as iron(0) donors? | ||
Recently, we have reported on the use [Fe(CO)3(PR3)] {R = Me (1a), Ph (1b), Cy (1c)} as Lewis bases in FLP chemistry where pronounced differences were observed based on the electron donating ability and steric profile of the phosphine substituents.3 Iron(0) complexes have also been used extensively as donors in coordination chemistry,1a notably as ligands for Cu, Ag, and Au complexes.4 However, gauging the donor strength of iron(0) (and other metal ligands) remains challenging. Previous efforts to determine the basicity and/or donor strength of iron(0) complexes have been crude, unreliable and/or analytically difficult.
Braunschweig used the transfer of the Lewis acid GaCl3 between Fe(0), Ru(0) and Pt(0) to gauge their relative Lewis basicity.5 He also applied the method pioneered by Gandon6 in measuring the hybridization of GaCl3 bound to the zero valent metal adducts to infer donor strength. Such an approach is contingent upon favourable formation and isolation of the GaCl3 adducts and their structural characterization. Additionally, such an approach may not reflect the behavior of the Fe(0) bases in solution where crystal packing effects manipulate the geometry of GaCl3. The Brønsted basicity of a large number of metal complexes has also been determined from the pKa of their conjugate acids (metal hydrides).7 Most pKa values are determined via equilibria with bases of known pKb, making determination of highly acidic metal hydrides difficult. This method is also imprecise, with error as high as 20%, and widely different values can be obtained depending on which yardstick base is used in the equilibrium. Further, established means to measure main group ligand donor strengths, such as the Tolman electronic parameter (TEP), Crabtree's modified electronic parameter and the Lever electronic parameter (LEP) are inept for weak and highly reductive donors such as Fe(0) complexes.8
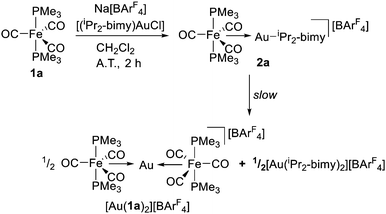 | ||
| Scheme 1 Formation of bimetallic complex 2a from 1a, [(iPr2-bimy) AuCl] and Na[BArF4]. 2a can further disproportionate into [Au(1a)2][BArF4] and [Au(iPr2-bimy)2][BArF4]. | ||
As an alternative to these methods (e.g. TEP, LEP) Huynh has reported on the correlation between ligand donor strengths and shift in the 13C NMR signal of a spectator ligand in complexes of the type [(iPr2-bimy)PdBr2(L)] and [(iPr2-bimy)Au(L)]+ (iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene, see Fig. 1(iii)).9 The 13C NMR signal of the benzimidazolinylidene carbon donor in [(iPr2-bimy)PdBr2(L)] corresponds to the Huynh electronic parameter (HEP) (where better donor ligands lead to lower field shifts), and Huynh has shown that the 13C carbenic NHC signal in [(iPr2-bimy)Au(L)]+ complexes can be directly correlated to the HEP for carbene ligands (this has yet to be expanded to other ligand classes). Given that Fe(0) has previously been reported to form complexes with gold fragments,4a,b we envisioned that the synthesis of bimetallic compounds of the type [(iPr2-bimy)Au–Fe(CO)3(PR3)2][BArF4] {BArF4 = tetrakis(pentafluorophenyl)borate} might allow an accurate ordering and comparison of the donor strengths of Fe(0) complexes as ligands.
To this end, we herein report on the formation and characterization of the Fe–Au adduct [(iPr2-bimy)Au–Fe(CO)3(PMe3)2][BArF4] (2a). We extend this class of complexes to [(iPr2-bimy)Au–Fe(CO)3(PR3)2][BArF4] (PR3 = PPh3, PCy3, PCyPh2, PMePh2, PMe2Ph, P(4-C6H4F)3) and find a correlation between their carbenic 13C NMR signal, their pKa and the TEP of the iron coordinated phosphine group. The ability to gauge the donor strength of iron(0) complexes (and potentially other donor metals) using this method circumvents the need to isolate unstable Fe–Au adducts and provides a high precision determination of relative Fe(0) donor strength.
Complex 2a was formed from mixing a solution of [Fe(CO)3(PMe3)2] (1a) with Na[BArF4] and [(iPr2-bimy)AuCl] in stoichiometric quantities (Scheme 1). The reaction was found to also form [Au(iPr2-bimy)2][BArF4] and [Au{Fe(CO)3(PMe3)2}2][BArF4] ([Au(1a)2][BArF4]), presumably via disproportionation of 2a. Nonetheless, compound 2a was found to be the predominant species formed with an NMR yield of 77% and could be isolated in 47% yield. The identity of [Au(1a)2][BArF4] and [Au(iPr2-bimy)2][BArF4] were confirmed via independent syntheses (see ESI†). Single crystal X-ray diffraction (SCXRD) analysis of [Au(1a)2][BArF4] (Fig. 2) revealed its structure to be closely related to the silver analogue [Ag{Fe(CO)3(PMe3)2}2][BArCl4] reported by Braunschweig.4g It was found that isolated samples of 2a dissolved in CH2Cl2 slowly formed [Au(1a)2][BArF4] and [Au(iPr2-bimy)2][BArF4] over a matter of hours.
Spectroscopic data for 2a reveal a decrease in the electron density and a reduction in symmetry at the iron centre, with ν(CO) shifting from 1871 cm−1 in 1a to 2005 cm−1, 1946 cm−1 and 1923 cm−1 in 2a (values in CH2Cl2 solution). And the 31P NMR signal arising from the PMe3 ligands shifting upfield from δP 38.4 in 1a to 23.6 in 2a. A signal at 190.1 ppm in the 13C NMR spectrum of 2a was identified as the iPr2-bimy carbenic resonance, with coupling to the iron bound PMe3 ligands observable (3JPC = 4.8 Hz, t, 2 P, CD2Cl2 solvent).
Compound 2a could be crystallized via diffusion of n-hexane into a saturated DCM solution at room temperature to generate crystals suitable for SCXRD. The molecular structure of 2a (Fig. 3) reveals a close Au–Fe contact at 2.562(1) Å. This distance is notably shorter than the M–Fe bond distances in [Au(1a)2][BArF4] (Au–Fe = 2.578(1) Å), implying a significant Au–Fe interaction in 2a. Indeed, evidence for a strong Fe–Au interaction can also be observed through a strong trans influence, elongating the Au–CNHC distance to 2.032(3) Å. This distance is longer than Au–CNHC distances observed in reported [Au(iPr2-bimy)(L)]+ complexes with weak doners, e.g. pyridine {Au–CNHC = 1.983(5) Å}, and similar to strong donors such as NHC ligands, e.g.iPr2-bimy {Au–CNHC = 2.023(6) Å}, iPr {Au–CNHC = 2.015(5) Å}.9c
The carbonyl environment around the iron centre in 2a fails to adopt an octahedral geometry, as is observed in other Fe(0)→M complexes,4 and two of the carbonyl ligands are bent towards the Au coordination site, with C–Fe–Au angles of 68.6(1)° and 73.8(1)°. Similar OC–Fe–CO geometries have been observed in Braunschweig's [Ag{Fe(CO)3(PMe3)2}2][BArCl4]4g and group 10 [Fe(CO)5] adducts,4a,b,e,f,h however, a DFT analysis by Frenking4b on [LAu–Fe(CO)5]+ systems (L = NHC or phosphine) concluded that there was little interaction between gold and the iron carbonyl ligands bent towards the gold centre despite the acute C–Fe–Au angles.
Huynh has found that HEP values correlate well to donor strength for σ-type donors, thus we aimed to understand the bonding between 1a and the ‘{(iPr2-bimy)Au}’ fragment and whether the carbonyl groups of 1a were involved in bonding with this fragment. To this end, we scrutinized the electronic structure of 2a using DFT calculations. Geometry optimization at the BP86-D3/def2-TZVP(+RECP on Au)/def-SVP level reproduced experimentally observed distances and νCO for 2a with high accuracy (Table S2, ESI†). The QTAIM analysis of the electron density does not reveal any bond critical points between Au and the in-plane CO carbon atoms, suggesting that the interaction is predominantly between Au and Fe, with little to no contribution from the carbonyl ligands (Fig. 4). Frenking's analysis of [LAu–Fe(CO)5]+ systems (L = NHC or phosphine) rendered a similar conclusion, with QTAIM parameters associated with 2a similar to those in Frenking's study.4b
The NOCV energy decomposition of the orbital interaction further supports this conclusion (Fig. S34, ESI†). The analysis reveals a dominant σ-type donor–acceptor interaction between the Fe centre and Au+ (6s/6p) with a much smaller π-interaction arising from backdonation from filled 5d AOs of Au+ to vacant carbonyl ligand molecular orbitals. Again, deformation densities and their respective fragment orbitals, as well as the associated interaction strength correlate strongly to Frenking's data. The σ-type interaction is also apparent from inspection of the MO diagram, where HOMO−1 shows relatively small contribution from the in-plane CO ligands. They are involved to some extent in some backbonding (which is very weak and only the dominant contribution is shown in Fig. S35, ESI†). The remainder of the MO diagram is unsurprising, and all d orbitals associated with the Au and Fe centres can be readily identified. The character of the vacant dz2 of Fe0 (d8) is smeared out over LUMO+2 and LUMO+3. DLPNO/CCSD(T) Local Energy Decomposition Analysis suggests a substantial binding energy of 66.7 kcal mol−1 between the two metal fragments (Table S3, ESI†). Interestingly, almost 33% of stabilization is due to London dispersion. Natural population analysis places a positive charge of +0.67e on Au.
Given the above conclusion, we proceeded to extend the measurement of 13C NMR iPr2-bimy carbenic signal values to other iron(0) complexes capable of acting as donors. To achieve this, we reacted Na[BArF20], [AuCl(iPr2-bimy)] and [Fe(CO)3(PR3)2] {PR3 = PPh3, PCy3, PCyPh2, PMePh2, P(4-C6H4F)3} together in CD2Cl2 then proceeded to measure their characteristic 13C NMR reporter signal. Huynh has established that a more positive (downfield) 13C NMR reporter signal for the iPr2-bimy ligand corresponds to a better σ-donor ligand.
It can be seen that the ordering of iron(0) ligands is as would be expected, with phosphino groups with more electron-donating substituents that generate a more electron rich Fe centre providing more positive 13C NMR values (Table 1). Notably, the ordering of the 13C NMR reporter signals of the ‘(iPr2-bimy)Au’ fragment follows the same ordering as the conjugate acid pKa values of the Fe(0) ligands (for those that are reported) with a strong linear relationship correlation (R2 = 0.9612, Fig. 5). It must be noted that in many instances the desired product was not dominant and/or stable (rendering isolation challenging). However, this NMR spectroscopic approach allowed us to gauge the donor strength of complexes 1 even when the target complexes 2 were in low concentration.10
| [Fe] donor complex | δ C | TEP7a of PR3 | [HFe]+ pKa6 |
|---|---|---|---|
| a 13C NMR signal of iPr2-bimy donor atom taken in CD2Cl2 (solvent reference 53.84 ppm).12 | |||
| Fe(CO)3(PMe3)2 (1a) | 190.1 | 2064.1 | 3.2–4 (DCE) |
| Fe(CO)3(PPh3)2 (1b) | 188.2 | 2068.9 | −0.6 to −1.1 (DCE) |
| Fe(CO)3(PCy3)2 (1c) | 190.7 | 2056.4 | 4.4 (DCE) |
| Fe(CO)3(PMePh2)2 (1d) | 188.4 | 2067.0 | 0.1–1 (DCE) |
| Fe(CO)3(PMe2Ph)2 (1e) | 189.5 | 2065.3 | 2.1 (DCE) |
| Fe(CO)3(PCyPh2)2 (1f) | 189.2 | n/a | 2 (DCE) |
| Fe(CO)3(P(4-C6H4F)3)2 (1g) | 187.0 | 2071.3 | n/a |
| Fe(CO)4(PPh3) (1h) | 185.4 | 2068.9 | n/a |
Further, we extended this technique to the tetracarbonyl iron(0) complex [Fe(CO)4(PPh3)] through 13C NMR analysis of [(iPr2-bimy)Au–Fe(CO)4(PPh3)][BArF4] (2h). As would be expected from the substitution of a phosphine with a more π-acidic ligand (i.e. CO), the 13C NMR reporter signal appeared at a higher field position (at 185.4 ppm), correlating with a poorer iron σ-donor. It is important to note that the high acidity of protonated [Fe(CO)4L] type complexes complicates their pKa determination,7 although Braunschweig has successfully applied his method of GaCl3 complexation to such complexes.5
13C NMR reporter signals for the ‘{(iPr2-bimy)Au}’ fragment with NHC, acyclic diamino carbene (ADC) and carbodicarbene (CDC) ligands have been reported.9c,11 Further, we recorded the 13C NMR reporter signals for [Au(iPr2-bimy)2][BArF4] and [(iPr2-bimy)Au(NC5H5)][BArF4] to be 187.5 and 168.3 ppm respectively (Table 1). Comparison of these data to 2a suggest that 1a has similar electron donation ability to NHCs on Au(I), and is a much better donor than pyridine. This is in agreement with hybridisation measurements of GaCl3 bound to 1a, NHCs and pyridine, and the large calculated Au–Fe binding energy (Table S3, ESI†).5a,6
In summary, we have synthesized and fully characterized the iron–gold adducts [(iPr2-bimy)Au–Fe(CO)3(PMe3)2][BArF4] (2a) and [Au–{Fe(CO)3(PMe3)2}2][BArF4] ([Au(1a)2]), where [Fe(CO)3(PMe3)2] (1a) acts as a ligand to the gold fragment. DFT supports the description of 1a as primarily a σ-donor to the gold centre in 2a, with only relatively little interaction between iron–carbonyl ligands and the gold centre, despite an acute Au–Fe–CO angle observed in the molecular structure of 2a. A correlation was found between the apparent donor strength of iron(0) complexes of the type [Fe(CO)3(PR3)2] (PR3 = PMe3, PPh3, PCy3, PCyPh2, PMePh2, P(4-C6H4F)3) and the carbenic 13C NMR signal of the ‘(iPr2-bimy)Au’ fragment in complexes of the type [(iPr2-bimy)Au–Fe(CO)3(PR3)2][BArF4]. This allows for a simple method to gauge donor strength in such Fe(0) complexes, and we will be exploring if this concept can be extended to other metal ligands capable of forming adducts with gold(I).
We thank the Singapore Agency for Science, Technology and Research for funding (A*STAR grant No. M21K2c0111). We acknowledge the Irish Centre for High-End Computing (ICHEC) for the provision of computational facilities and technical support.
Conflicts of interest
The authors declare no competing financial interest.References
- (a) J. Bauer, H. Braunschweig and R. D. Dewhurst, Chem. Rev., 2012, 112, 4329 CrossRef CAS PubMed; (b) N. Hidalgo, M. G. Alférez and J. Campos, Frustrated Lewis Pairs Based on Transition Metals, in Frustrated Lewis Pairs, ed. C. J. Slootweg and A. R. Jupp, Springer: Cham, 2021, vol. 2, pp. 319–359 Search PubMed.
- (a) N. P. Mankad, Catalysis with multinuclear complexes, in Non-noble metal catalysis molecular approaches and reactions, ed. R. J. M. Klein Gebbink and M.-E. Moret, Wiley-VCH, Weinheim, Germany, 2019, pp. 49–68 Search PubMed; (b) I. G. Powers and C. Uyeda, ACS Catal., 2017, 7, 936 CrossRef CAS; (c) W. Xu, L. Qiao and J. Xie, Chem. Commun., 2020, 56, 8524 RSC.
- (a) H. Tinnermann, C. Fraser and R. D. Young, Dalton Trans., 2020, 49, 15184 RSC; (b) H. Tinnermann, S. Sung, D. Csókás, Z. H. Toh, C. Fraser and R. D. Young, J. Am. Chem. Soc., 2021, 143, 10700 CrossRef CAS PubMed.
- For recent examples see: (a) G. Wang, T. T. Ponduru, Q. Wang, L. Zhao, G. Frenking and H. V. R. Dias, Chem. – Eur. J., 2017, 23, 17222 CrossRef CAS PubMed; (b) S. Pan, S. M. N. V. T. Gorantla, D. Parasar, H. V. R. Dias and G. Frenking, Chem. – Eur. J., 2021, 27, 6936 CrossRef CAS PubMed; (c) B. Berti, M. Bortoluzzi, C. Cesari, C. Femoni, M. C. Iapalucci, R. Mazzoni, F. Vacca and S. Zacchini, Eur. J. Inorg. Chem., 2019, 3084 CrossRef CAS; (d) B. Berti, M. Bortoluzzi, C. Cesari, C. Femoni, M. C. Iapalucci, R. Mazzoni, F. Vacca and S. Zacchini, Inorg. Chem., 2019, 58, 2911 CrossRef CAS PubMed; (e) G. Wang, A. Noonikara-Poyil, I. Fernández and H. V. R. Dias, Chem. Commun., 2022, 58, 3222 RSC; (f) G. Wang, Y. S. Ceylan, T. R. Cundari and H. V. R. Dias, J. Am. Chem. Soc., 2017, 139, 14292 CrossRef CAS PubMed; (g) H. Braunschweig, R. D. Dewhurst, F. Hupp and C. Schneider, Chem. Commun., 2014, 50, 15685 RSC; (h) P. J. Malinowski and I. Krossing, Angew. Chem., Int. Ed., 2014, 53, 13460 CrossRef CAS PubMed.
- (a) H. Braunschweig, R. D. Dewhurst, F. Hupp, C. Kaufmann, A. K. Phukan, C. Schneider and Q. Ye, Chem. Sci., 2014, 5, 4099 RSC; (b) H. Braunschweig, C. Brunecker, R. D. Dewhurst, C. Schneider and B. Wennemann, Chem. – Eur. J., 2015, 21, 19195 CrossRef CAS PubMed; (c) B. Demerseman, G. Bouquet and M. Bigorgne, J. Organomet. Chem., 1972, 35, 341 CrossRef CAS.
- A. El-Hellani, J. Monot, S. Tang, R. Guillot, C. Bour and V. Gandon, Inorg. Chem., 2013, 52, 11493 CrossRef CAS PubMed.
- (a) R. H. Morris, Chem. Rev., 2016, 116, 8588 CrossRef CAS PubMed; (b) R. H. Morris, J. Am. Chem. Soc., 2014, 136, 1948 CrossRef CAS PubMed.
- (a) C. A. Tolman, J. Am. Chem. Soc., 1970, 92, 2953 CrossRef CAS; (b) A. R. Chianese, X. Li, M. C. Janzen, J. W. Faller and R. H. Crabtree, Organometallics, 2003, 22, 1663 CrossRef CAS; (c) A. B. P. Lever, Inorg. Chem., 1990, 29, 1271 CrossRef CAS.
- (a) Q. Teng and H. V. Huynh, Dalton Trans., 2017, 46, 614 RSC; (b) H. V. Huynh, Y. Han, R. Jothibasu and J. A. Yang, Organometallics, 2009, 28, 5395 CrossRef CAS; (c) S. Guo, H. Sivaram, D. Yuan and H. V. Huynh, Organometallics, 2013, 32, 3685 CrossRef CAS; (d) H. V. Huynh, Chem. Lett., 2021, 50, 1831 CrossRef CAS.
- For low concentration samples [13C] iPr2-bimy was used.
- (a) X. Xu, Z. Zhang, S. Huang, L. Cao, W. Liu and X. Yan, Dalton Trans., 2019, 48, 6931 RSC; (b) C. Singh, A. Kumar and H. V. Huynh, Inorg. Chem., 2020, 59, 8451 CrossRef CAS PubMed; (c) S.-k Liu, W.-C. Chen, G. P. A. Yap and T.-G. Ong, Organometallics, 2020, 39, 4395 CrossRef CAS.
- G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw and K. I. Goldberg, Organometallics, 2010, 29, 2176 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2206892 and 2206893. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc05041b |
| This journal is © The Royal Society of Chemistry 2022 |