Guest-activated quaternary ammonium salt hosts emit room temperature phosphorescence†
Xinyue
Xu
,
Zehang
Chen
,
Yunxiang
Lei
 *,
Xinyu
Sun
,
Miaochang
Liu
*,
Xinyu
Sun
,
Miaochang
Liu
 ,
Huayue
Wu
,
Huayue
Wu
 and
Xiaobo
Huang
and
Xiaobo
Huang
 *
*
College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, 325035, P. R. China. E-mail: yunxianglei@wzu.edu.cn; xiaobhuang@wzu.edu.cn
First published on 5th September 2022
Abstract
A novel doped system based on quaternary ammonium salts as hosts was established. Interestingly, it is the guest-activated hosts that emit room temperature phosphorescence, rather than the host-assisted guests in traditional doped systems.
The host–guest doping strategy has gradually become the main choice for the construction of organic small molecule room temperature phosphorescence/RTP materials due to its convenient preparation and high success rate.1 In the doped system, a small amount of the guest is doped into the host matrix, and the guest acts as an emitter while the host provides an environment conducive to the phosphorescence emission of the guest.2 The types and phosphorescence properties of doped materials have been greatly improved and enriched; however, the luminescence mechanism of host–guest doped materials still needs further in-depth research. Although it has been found that in most doped materials, the excitons of guest molecules undergo intersystem crossing/ISC and eventually emit phosphorescence, the complex role played by the host matrix in addition to providing a rigid environment is still controversial. Overall, there are four proposed mechanisms for the role of the host matrix in the doped system. First, the host matrix absorbs the excitation source energy and then transfers the excitons to the guest through Förster energy transfer or Dexter energy transfer.3 Second, the triplet state of the host acts as a bridge to facilitate the transfer of excitons from the singlet state of the guest to the triplet state of the guest.4 Third, the dipole–dipole interaction between the singlet states of the host–guest facilitates the ISC of excitons.1e Fourth, intermolecular charge transfer or charge recombination occurs between the host and guest.1c Most host matrices are neutral organics, while charged organic salts are rarely used as hosts. However, charged organic salts easily interact with the excitons of organic compounds,5 which not only produces special or unexpected phosphorescence properties but also facilitates the exploration of the role of the host matrix in the doped system.
In this work, we first synthesized a large number of four quaternary ammonium salt organics as the host through a simple one-step reaction design, and using 2-hydroxy-4-(2-hydroxyprop-1-en-1-yl)-6-methyl-isophthalonitrile/Mb as a guest to construct a new doped system. The doped materials have a strong cyan-green afterglow time of up to eight seconds, the phosphorescence lifetime is 581–1091 ms, and the phosphorescence quantum yield is 14.7–21.2%. Interestingly, the experimental results show that in the doped system, although the guest molecules still absorb the energy, it is the hosts that eventually emit the phosphorescence. To further verify this phenomenon, three compounds with different phosphorescence wavelengths were further selected as auxiliary guests. The results show that the phosphorescence wavelength of the doped material is still around 500 nm even if the pyrene derivative with a phosphorescence wavelength of 650 nm acts as a guest. Based on this, we propose a new luminescence mechanism for doped materials, that is, the guest molecules absorb energy first, and then the singlet excitons of the guest are still transferred to the triplet state of the host. The charge carried by the host will stabilize the triplet excitons; therefore, the triplet excitons of the host directly transition back to the ground state to generate phosphorescence. This work has important reference significance for expanding and enriching the luminescence properties and luminescence mechanisms of doped materials.
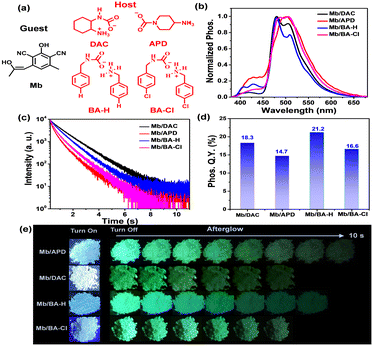
The guest Mb is obtained in two steps according to the reported synthetic method (Fig. 1(a) and Scheme S1, ESI†). High performance liquid chromatography showed that the guest was of high purity (Fig. S1, ESI†). The guest exhibits strong cyan fluorescence in toluene solution with an emission wavelength of 488 nm and a fluorescence quantum efficiency of 67% (Fig. S2, ESI†); additionally, the fluorescence wavelength of the guest did not show a red-shift phenomenon from low-polarity solvent to high-polarity solvent (Fig. S1, ESI†), indicating that the guest did not undergo intramolecular charge transfer. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of Mb are almost identical, both distributed throughout the molecule, and the energy gap between the HOMO and LUMO is 4.27 eV (Fig. S3, ESI†). Four quaternary ammonium salt hosts are obtained through a simple one-step reaction (Fig. 1(a) and Scheme S2, ESI†), two of which are aliphatic. (DAC, APD) and the other two are aromatic (BA-H, BA-Cl). HPLC showed that the compounds were of high purity (Fig. S4, ESI†). The solid-state hosts have weak cyan fluorescence with an emission wavelength of around 450 nm (Fig. S5, ESI†), but almost no RTP properties (Fig. S6, ESI†). The doped materials are prepared by the solvent evaporation method, that is, the corresponding amount of the guest and the host are dissolved in dichloromethane and methanol, respectively, and then the solvent is distilled under reduced pressure with an oil pump to obtain the mixture. In the doped system, the content of the guest is closely related to the phosphorescence properties; therefore, a series of Mb/BA-H doped materials with different guest–host molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20–1
20–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) are prepared. The results show that when the ratio of guest to host is 1
000) are prepared. The results show that when the ratio of guest to host is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, the doped material has the strongest phosphorescence emission (Fig. S7, ESI†). Therefore, the host–guest molar ratios of the remaining doped materials are all 1
100, the doped material has the strongest phosphorescence emission (Fig. S7, ESI†). Therefore, the host–guest molar ratios of the remaining doped materials are all 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.
100.
All doped materials showed strong turquoise afterglow after the excitation lamp was removed, and the afterglow times were as long as 7–10 s (Fig. 1(e)), indicating that the doped materials had excellent RTP activity. Delayed emission spectra further show that the emission peaks of the four doped materials are almost all around 500 nm (Fig. 1(b)). In addition, the Commission Internationale de l’Eclairage/CIE coordinates of the four doped materials are around 0.18–0.21, 0.38–0.44, which also means that the phosphorescent colors are cyan-green (Fig. S8, ESI†). The delayed emission decay curves show that the doped materials have long phosphorescence lifetimes, with lifetimes of 1091 ms, 581 ms, 823 ms and 680 ms for Mb/DAC, Mb/APD, Mb/BA-H and Mb/BA-Cl, respectively (Fig. 1(c)).
Additionally, the phosphorescence quantum yields/Q.Y. show that the doped materials also have satisfactory luminescence intensity, and the phosphorescence Q.Y. of Mb/DAC, Mb/APD, Mb/BA-H and Mb/BA-Cl is 18.3%, 14.7%, 21.2%, and 16.6%, respectively (Fig. 1(d)). There are two kinds of persistent afterglow luminescence (RTP type and TADF type) of doped materials;1e,2 in order to accurately describe the afterglow type of this doped system, the delayed emission intensity of Mb/BA-Cl at different temperatures was tested. The results show that the emission intensity of Mb/BA-Cl decreases significantly with the increase in temperature; as the temperature increases from 77 K to 297 K, the delayed emission intensity of Mb/BA-Cl gradually decreases, and the magnitude of attenuation reaches 6.3 times (Fig. S9, ESI†), which indicates that the afterglow of this doped system is RTP type. In addition, the XRD curves show that the hosts are crystalline, and the morphology of the hosts does not change significantly before and after doping (Fig. S10, ESI†). Considering that the hosts are salts, the stability of the doped materials to water was studied, and the results showed that even if the doped material Mb/BA-Cl were soaked in water for 24 hours, there was still a bright green afterglow (Fig. S11, ESI†).
In most doped systems, the phosphorescence of the materials is actually ultimately emitted by the guest molecules.2 In order to verify whether the phosphorescence of this doped system is also emitted by the guest, the phosphorescence spectra of the aggregate state guest and four solid state hosts at low temperature were tested.
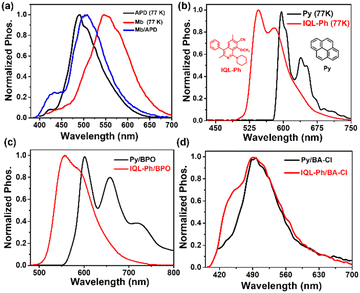
It is worth mentioning that the content of the guest in this doped system is relatively high and the solvent evaporation method is used to prepare the doped materials. Therefore, we believe that the guest is present in the host matrix as aggregates (rather than individual molecules).6 The results have shown that the guest has a strong yellow-orange afterglow at low temperature, and the phosphorescence wavelength is around 555 nm (Fig. S12, ESI†). However, the four hosts have strong cyan-green phosphorescence with an emission wavelength of around 500 nm (Fig. S13, ESI†). What is even more surprising is that the phosphorescence spectra of the doped materials are almost completely coincident with those of the hosts, which means that the phosphorescence of the doped materials is emitted by the host molecules. (Fig. 2(a)). To carefully verify this anomaly, two compounds Py and IQL-Ph with phosphorescence wavelengths of 620 nm and 565 nm were selected as auxiliary guests (Fig. 2(b)). It has been confirmed in our previous work that the phosphorescence wavelengths of the doped materials obtained by doping these two auxiliary guests into the neutral host (benzophenone/BPO) are basically consistent with those of the guests (Fig. 2(c)). However, the phosphorescence wavelengths of the doped materials obtained by doping these two auxiliary guests into the salt host/BA-Cl are all around 500 nm (Fig. 2(d)). The above results indicate that the phosphorescence in this doped system with quaternary ammonium salt as the host is indeed emitted by the host molecules. In other words, a small amount of guest activates the quaternary ammonium salts host to have RTP properties, rather than the guest emitting phosphorescence in the host matrix.
Determining whether the host or the guest absorbs the excitation light is the premise of studying the luminescence mechanism of doped materials. Therefore, the absorption wavelengths of the guest and hosts in the solid state were tested first. As shown in Fig. S14a (ESI†), the maximum absorption wavelength of Mb is 386 nm, while that of the hosts is less than 300 nm. Similarly, the excitation wavelength of the guest is 390 nm, and although the hosts have a weak shoulder peak at 300–350 nm, the absorption intensity is much lower than that of the guest (Fig. S14b, ESI†). Next, the excitation wavelengths of the doped materials were tested, and the results showed that the maximum excitation wavelength of the materials was around 385 nm (Fig. S14c, ESI†). Further excitation-dependent experiments also show that the doped materials have weak phosphorescence intensity under excitation sources of 300–320 nm, and the phosphorescence intensity is the strongest at excitation wavelengths of 370–390 nm (Fig. S14d, ESI†). It should be pointed out that even if the excitation wavelength reaches 400 nm (the hosts can hardly absorb the energy of this band), the doped materials still have strong phosphorescence activity (Fig. S14d and S15, ESI†). The above results fully show that the excitation source energy of this doped system is also absorbed by the guest molecules first, which is consistent with most doped materials with neutral compounds as the host.
To sum up, in the doped system with quaternary ammonium salts as the hosts, the guest molecules absorb energy first, and the host molecules finally emit phosphorescence. Recently, researchers have developed a doped system with salt compounds as the hosts, and reported for the first time that the phosphorescence of the doped system is emitted by the host matrix.7 Due to the strong electron acceptor properties of the hosts and the strong electron-donating properties of the guest, the formation of intermolecular charge transfer/ICT between the host and the guest is considered to be the cause of this phenomenon. In some doped systems with neutral compounds as the host, the formation of CT states or charge recombination between the host and guest is considered to be the inducement for the RTP activity of doped materials.8 Therefore, in order to verify whether the formation of ICT between the host and guest leads to the RTP activity of the materials in our doped system, two compounds N,N,N′,N′-tetramethylbenzidine/TMB and N,N,N′,N′-tetraphenylbenzidine/TPB with strong electron donating properties and already proven to be excellent guests were selected as control guests (Fig. S16a, ESI†).8a,9 The results show that both doped materials TMB/BA-Cl and TPB/BA-Cl have no RTP activity (Fig. S16b, ESI†). Furthermore, neither the guest Mb nor the auxiliary guests Py and IQ-Ph have strong electron-donating properties, and the absorption spectra also showed that the doped material did not form a new absorption peak representing the CT state (Fig. S16c and S17, ESI†); therefore, the ICT between the host–guest may not be the main reason for the RTP property of our doped system. In previous work, it was considered that the host matrix assists the ISC of the guest excitons. As shown in Fig. 3(a), the singlet excitons are transferred to the triplet state of the host after the guest absorbs the energy of the excitation source, and then the host transfers the excitons back to the triplet state of the guest, and finally the guest emits phosphorescence.4 In this doped system, the guest still absorbs energy but the host finally emits phosphorescence. Therefore, it is still believed that the guest transfers excitons to the triplet state of the host, while the host does not transfer excitons back to the guest (Fig. 3(b)). Instead, it directly radiates room temperature phosphorescence. This may be due to the special interaction between the charges of the ionic compounds and the excitons, thereby stabilizing the triplet excitons. For example, researchers often use ionic compounds as dopants to prepare organic electroluminescent materials and organic conductive materials.5 In addition, the single crystal structures of the hosts further indicate the existence of strong interactions between molecules. As shown in Fig. 3(d), the positively charged tertiary nitrogen and the surrounding negatively charged oxygen atoms form three strong hydrogen bonds with the distance of only 2.61–2.91 Å, and these hydrogen bonds are beneficial to stabilize triplet excitons. In order to more realistically reflect the energy gap between the host and guest, the corresponding energy gap was calculated using the emission spectra (fluorescence and phosphorescence) of the host and guest at low temperature. The low-temperature (77 K) fluorescence wavelength of the guests (Mb, Py, and IQL-Ph) is 405–475 nm (Fig. S18, ESI†), and their phosphorescence wavelength is 550–620 nm (Fig. 2(b)), while the phosphorescence wavelength of the hosts is around 500 nm (Fig. S13, ESI†). The phosphorescence lifetimes of Py/BA-Cl and IQL-Ph/BA-Cl are 432 ms and 537 ms, respectively (Fig. S19, ESI†). The calculation results show that the ΔEST values (band gaps between S1 and T1) of Mb, IQL-Ph, and Py are 0.34 eV, 0.52 eV, and 1.06 eV, respectively, and such large ΔEST makes it difficult for excitons to undergo intersystem crossing. However, the ΔEST values (band gaps between S1 of the guest and T1 of the host) of Mb/BA-Cl, IQL-Ph/BA-Cl, and Py/BA-Cl are 0.11 eV, 0.24 eV, and 0.58 eV, respectively, and the small ΔEST is obviously more conducive to the ISC of excitons (Fig. 3(c)).
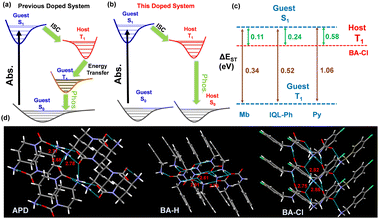 | ||
| Fig. 3 (a) Proposed luminescence mechanism of the previous doped system. (b) Proposed luminescence mechanism of this doped system. (c) ΔEST of the guest and the hosts. (d) Single crystal structures of APD, BA-H, and BA-Cl (the cyan dotted lines represent hydrogen bonds, and the red numbers represent the distance of hydrogen bonds. CCDC: 2189315 of APD, 2189318 of BA-H, 2189320 of BA-Cl).† | ||
In this work, four novel quaternary ammonium salt hosts were synthesized through a simple one-step reaction, and a new doped system was constructed with Mb as the guest. The phosphorescence lifetime of the doped materials is 581–1091 ms, and the phosphorescence efficiency is 14.2–21.2%. More importantly, unlike most doped systems, although the guest still absorbs the energy of the excitation source, the phosphorescence of this doped system is ultimately emitted by the host rather than the guest. The proposed mechanism suggests that the guest transfers the excitons to the host, and then the charge of the host interacts with the triplet excitons, finally resulting in the host having RTP activity. This work is of great significance for enriching and expanding the systems and mechanisms of organic doped phosphorescence materials.
This work was supported by the National Natural Science Foundation of China (No. 22105148 and 22071184) and the Zhejiang Provincial Natural Science Foundation of China (No. LY20B020014).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) W. J. Zhao, Z. K. He and B. Z. Tang, Nat. Rev. Mater., 2020, 5, 869–885 CrossRef CAS; (b) R. Gao, M. S. Kodaimati and D. P. Yan, Chem. Soc. Rev., 2021, 50, 5564 RSC; (c) B. B. Ding, L. W. Ma, Z. Z. Huang, X. Ma and H. Tian, Sci. Adv., 2021, 7, eabf9668 CrossRef CAS PubMed; (d) B. Chen, W. H. Huang, X. C. Nie, F. Liao, H. Miao, X. P. Zhang and G. Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 16970–16973 CrossRef CAS; (e) X. P. Wang, Y. Sun, G. M. Wang, J. Y. Li, X. Li and K. K. Zhang, Angew. Chem., Int. Ed., 2021, 60, 17138–17147 CrossRef CAS PubMed; (f) D. K. Jia, H. Zhong, S. X. Jiang, R. S. Yao and F. Wang, Chin. Chem. Lett., 2022, 02, 081 Search PubMed; (g) X. Zhang, H. Ma, H. Shi, H. Wang, A. Lv, L. Bian, M. Zhang, C. Ma, K. Lin, M. Gu, Z. An, X. Liu and W. Huang, Nat. Mater., 2021, 20, 1539–1544 CrossRef PubMed; (h) K. J. Chen, Y. T. Jiang, Y. B. Zhu, Y. X. Lei, W. B. Dai, M. C. Liu, Z. X. Cai, H. Y. Wu, X. B. Huang and Y. P. Dong, J. Mater. Chem. C, 2022, 10, 11607–11613 RSC.
- (a) J. B. Wei, B. Y. Liang, R. H. Duan, Z. Cheng, C. L. Li, T. L. Zhou, Y. P. Yi and Y. Wang, Angew. Chem., Int. Ed., 2016, 55, 15589–15593 CrossRef CAS PubMed; (b) X. Q. Liu, W. B. Dai, J. J. Qian, Y. X. Lei, M. C. Liu, Z. X. Cai, X. B. Huang, H. Y. Wu and Y. P. Dong, J. Mater. Chem. C, 2021, 9, 3391–3395 RSC; (c) Y. Guo, K. J. Chen, Z. C. Hu, Y. X. Lei, X. Q. Liu, M. C. Liu, Z. X. Cai, J. W. Xiao, H. Y. Wu and X. B. Huang, J. Phys. Chem. Lett., 2022, 13, 7607–7617 CrossRef CAS; (d) X. P. Zhang, L. L. Du, W. J. Zhao, Z. Zhao, Y. Xiong, X. W. He, P. F. Gao, P. Alam, C. Wang, Z. Li, J. Leng, J. X. Liu, C. Y. Zhou, J. W. Y. Lam, D. L. Phillips, G. Q. Zhang and B. Z. Tang, Nat. Commun., 2019, 10, 5161 CrossRef PubMed; (e) N. N. Liu, Y. Y. Pan, Y. X. Lei, M. C. Liu, C. D. Peng, Z. X. Cai, G. M. Shen, H. Y. Wu, X. B. Huang and Y. P. Dong, Chem. Eng. J., 2022, 433, 133530 CrossRef CAS.
- (a) B. Zhou and D. P. Yan, Adv. Funct. Mater., 2019, 29, 1807599 CrossRef; (b) Y. S. Wang, J. Yang, M. M. Fang, Y. S. Yu, B. Zou, L. W. Wang, Y. Tian, J. X. Cheng, B. Z. Tang and Z. Li, Matter, 2020, 3, 449–463 CrossRef.
- (a) X. Y. Zhang, D. Wang, Y. X. Lei, M. C. Liu, Z. X. Cai, H. Y. Wu, G. M. Shen, X. B. Huang and Y. P. Dong, Chem. Commun., 2022, 58, 1179–1182 RSC; (b) W. B. Dai, X. W. Niu, X. X. Wu, Y. Ren, Y. F. Zhang, G. C. Li, H. Su, Y. X. Lei, J. W. Xiao, J. B. Shi, B. Tong, Z. X. Cai and Y. P. Dong, Angew. Chem., Int. Ed., 2022, 61, e202200236 CAS; (c) Y. X. Lei, W. B. Dai, J. X. Guan, S. Guo, F. Ren, Y. D. Zhou, J. B. Shi, B. Tong, Z. X. Cai, J. R. Zheng and Y. P. Dong, Angew. Chem., Int. Ed., 2020, 59, 16054–16060 CrossRef CAS; (d) Z. L. Xie, X. Y. Zhang, H. L. Wang, C. Huang, H. D. Sun, M. Y. Dong, L. Ji, Z. F. An, T. Yu and W. Huang, Nat. Commun., 2021, 12, 3522 CrossRef CAS; (e) F. M. Xiao, H. Q. Gao, Y. X. Lei, W. B. Dai, M. C. Liu, X. Y. Zheng, Z. X. Cai, X. B. Huang, H. Y. Wu and D. Ding, Nat. Commun., 2022, 13, 186 CrossRef CAS PubMed.
- (a) X. H. Wu, A. Surendran, J. Ko, O. Filonik, E. M. Herzig, P. Müller-Buschbaum and W. L. Leong, Adv. Mater., 2019, 31, 1805544 CrossRef; (b) C. Z. Shi, L. H. Qiu, X. J. Chen, H. G. Zhang, L. Wang and F. Yan, ACS Appl. Mater. Interfaces, 2013, 5, 1453–1459 CrossRef CAS PubMed; (c) Y. Khan, Y. Ahn, J. Hwa Seo and B. Walker, J. Mater. Chem. C, 2020, 8, 13953–13971 RSC.
- X. Q. Liu, Y. Y. Pan, Y. X. Lei, N. N. Liu, W. B. Dai, M. C. Liu, Z. X. Cai, H. Y. Wu, X. B. Huang and Y. P. Dong, J. Phys. Chem. Lett., 2021, 12, 7357–7364 CrossRef CAS PubMed.
- X. J. Deng, J. Huang, G. M. Wang, J. Y. Li, X. Li, C. H. Lei and K. K. Zhang, Chem. Commun., 2022, 58, 8137 RSC.
- (a) R. Kabe and C. Adachi, Nature, 2017, 550, 384–387 CrossRef CAS PubMed; (b) C. J. Chen, Z. G. Chi, K. C. Chong, A. S. Batsanov, Z. Yang, Z. Mao, Z. Y. Yang and B. Liu, Nat. Mater., 2021, 20, 175–180 CrossRef CAS PubMed.
- (a) Y. L. Ning, J. F. Yang, H. Si, H. Z. Wu, X. Y. Zheng, A. J. Qin and B. Z. Tang, Sci. China: Chem., 2021, 64, 739–744 CrossRef CAS; (b) P. Alam, N. L. C. Leung, J. K. Liu, T. Shing Cheung, X. P. Zhang, Z. K. He, R. T. K. Kwok, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, C. C. S. Chan, K. Sing Wong, Q. Peng and B. Z. Tang, Adv. Mater., 2020, 32, 2001026 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2189315 of APD, 2189318 of BA-H and 2189320 of BA-Cl. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc04521d |
| This journal is © The Royal Society of Chemistry 2022 |