Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation of new crystalline qtz-[Zn(mIm)2] polymorph from amorphous ZIF-8†
Michael F.
Thorne‡
 a,
Celia
Castillo-Blas‡
a,
Celia
Castillo-Blas‡
 a,
Lauren N.
McHugh
a,
Lauren N.
McHugh
 a,
Alice M.
Bumstead
a,
Alice M.
Bumstead
 a,
Georgina
Robertson
a,
Adam F.
Sapnik
a,
Georgina
Robertson
a,
Adam F.
Sapnik
 a,
Chloe S.
Coates
a,
Chloe S.
Coates
 b,
Farheen N.
Sayed
b,
Farheen N.
Sayed
 b,
Clare P.
Grey
b,
Clare P.
Grey
 b,
David A.
Keen
b,
David A.
Keen
 c,
Martin
Etter
d,
Ivan
da Silva
c,
Martin
Etter
d,
Ivan
da Silva
 c,
Krunoslav
Užarević
c,
Krunoslav
Užarević
 e and
Thomas D.
Bennett
e and
Thomas D.
Bennett
 *a
*a
aDepartment of Materials Science and Metallurgy, University of Cambridge, 27 Charles Babbage Road, Cambridge, CB3 0FS, UK. E-mail: tdb35@cam.ac.uk
bYusuf Hamied Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK
cISIS Facility, STFC Rutherford Appleton Laboratory, Chilton, Didcot OX11 0QX, UK
dDeutsches Elektronen Synchrotron, FS-PETRA-D, P02.1, Notkestr. 85, 22607, Hamburg, Germany
eRuđer Bošković Institute, Bijenička Cesta 54, Zagreb, HR-10000, Croatia
First published on 30th September 2022
Abstract
The structure of a new ZIF-8 polymorph with quartz topology (qtz) is reported. This qtz-[Zn(mIm)2] phase was obtained by mechanically amorphising crystalline ZIF-8, before heating the resultant amorphous phase to between 282 and 316 °C. The high-temperature phase structure was obtained from powder X-ray diffraction, and its thermal behaviour, CO2 gas sorption properties and dye adsorption ability were investigated.
Zeolitic imidazolate frameworks (ZIFs) are a subclass of metal–organic frameworks (MOFs) which contain M2+ cations, often Zn2+, linked by imidazolate anions.1,2 ZIFs have been noted for their exceptionally high thermal stability with respect to other MOFs,3 and have potential applications in gas separation, chemical sensing, drug delivery and catalysis.4
The rich energetic landscape of ZIFs leads to interesting polymorphic behaviour and amorphisation.5 ZIFs are typically prepared as crystalline powders. Amorphous MOFs have gained, however, great attention in recent years. They retain the building blocks and connectivity of their crystalline counterparts,6 though they lack long-range order. Despite this, they possess very promising optical and mechanical properties for practical applications.7
The amorphous phase of ZIFs (aZIFs) can be accessed by melt-quenching,8 thermal amorphisation of a crystalline ZIF,9 or, more universally, by mechanical milling.6 Melting or thermal amorphisation of crystalline ZIFs is, however, somewhat challenging and is therefore not always a widely applicable approach.10 The low density of many crystalline ZIFs means the temperature required to access the liquid state is far above their decomposition temperatures (Td). This is due to the high free energy barrier for the decoordination and reattachment of imidazolate linkers from the metal cations during melting.10,11 On the other hand, mechanical amorphisation is a reliable way to obtain amorphous ZIFs, and is typically achieved within one hour of milling.12,13 Amorphisation of ZIF-4, [Zn(Im)2] – where Im = imidazolate (C3N2H3)−, for example, occurs in under 30 minutes. The amorphous product, amZIF-4, curiously undergoes crystallisation to the dense ZIF-zni phase upon heating to 475 °C.14,15
Mechanical amorphisation has also been reported for amZIF-62 [Zn(Im)2−x(bIm)x] and amZIF-UC-5 [Zn(Im)2−x(ClbIm)x] materials, where bIm = benzimidazolate (C7N2H5)−, and ClbIm = 5-chlorobenzimdazolate (C7N2H4Cl)−.16 The inclusion of substituted imidazolate linkers, such as bIm and ClbIm, traps the amZIFs in a metastable amorphous phase upon heating.16 The crystallisation of amZIF-4 and the glass transition temperature (Tg) of amZIF-62 highlight the importance of investigating the thermal response of aZIFs in general, as it may be possible to induce similar transitions in other aZIFs. Tg is the temperature in amorphous materials where the reversible transition between a glassy state and a viscoelastic state occurs.
ZIF-8 [Zn(mIm)2], mIm = 2-methylimidazolate (C4N2H5)− with sodalite (SOD) topology, is a widely studied, prototypical ZIF.17–19 ZIF-8 is of great interest due to its high porosity and relatively high thermal stability compared to most MOFs.3 Further to this, ZIF-8 is one of the few commercially-manufactured ZIFs, being sold under the name Basolite® Z1200.20
Mechanical amorphisation of ZIF-8 has been previously reported in a vibratory ball mill, yielding amZIF-8.21 X-ray total scattering experiments and subsequent refinements using Reverse Monte Carlo modelling revealed amZIF-8 retains the short-range order of ZIF-8 [Zn(mIm)2] whilst displaying a continuous random network (CRN) topology.21 The thermal stability compared to mass loss of amZIF-8 was also investigated, using thermogravimetric analysis (TGA).21 This revealed a decrease in thermal stability at elevated temperatures with respect to crystalline ZIF-8. No other thermal measurements were performed.
Furthermore, ZIF-8 is able to transform into other crystalline polymorphs by mechanical milling. For example, milling for 35 minutes results in sequential crystallisation of amZIF-8 to the relatively dense [Zn(mIm)2] polymorphs kat-[Zn(mIm)2] and dia-[Zn(mIm)2], where kat and dia refer to the topologies of the new crystalline phases.22 This extended milling time of amZIF-8 is analogous to heating amZIF-4,14 where energy is supplied to the metastable amorphous phase, allowing crystallisation to ZIF-zni to occur. This suggests that the additional energy supplied by milling facilitates linker mobility, and therefore potential phase transitions.
While the thermal stability of MOFs is generally determined by TGA, this technique provides no information on potential phase changes that may occur during heating. Therefore, other techniques such as DSC should be performed to identify potential new phases.23
Here we investigate whether any additional phases are formed upon heating amZIF-8, using DSC and TGA. First, crystalline ZIF-8 was synthesised following the reported methodology.19 Powder X-ray diffraction (PXRD) was performed on the resulting white solid in order to confirm the phase purity through a Pawley refinement (Fig. S1 and Table S1, ESI†).24 ZIF-8 was then mechanochemically amorphised in a vibratory ball mill (see Methods). In situ monitoring of the process by synchrotron PXRD confirmed direct amorphisation of ZIF-8 was completed after 40 minutes of ball milling to yield amZIF-8. The lack of unassigned Bragg peaks also confirms that the amorphisation process occurred without the formation of any new phases (Fig. 1a and Fig. S2, ESI†). Whilst heating to 1000 °C, amZIF-8 showed major decomposition above 400 °C (Fig. S3, ESI†). Guided by this, DSC analysis of amZIF-8 under an argon atmosphere was only performed up to 400 °C, where a single exothermic transition was observed (Fig. 1b). The onset of this event occurred at 282.0 °C, i.e. before significant mass loss occurred and it corresponds to an enthalpy change of −22.3 J g−1 (−5.31 kJ mol−1) (Fig. 1b and Fig. S4, ESI†). After heating the sample to 400 °C and subsequently cooling to room temperature, PXRD confirmed the formation of a crystalline phase (Fig. 1c and Fig. S4, ESI†). Therefore, the exothermic transition may be denoted as Tx (= 282 °C), where ΔHx = −5.31 kJ mol−1 and ΔSx = −9.14 J mol−1 K−1 (Fig. 1b). The Tx at elevated temperatures highlights the metastability of amorphous ZIFs compared to their crystalline counterparts, as a significant amount of energy is released from amZIF-8 upon crystallisation. Surprisingly, using the same DSC experimental conditions, this phase transition was not detected under a nitrogen atmosphere.
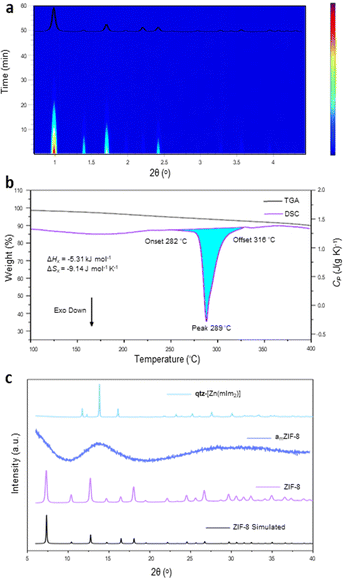 | ||
| Fig. 1 (a) In situ PXRD ball milling amorphisation of ZIF-8 showing complete amorphisation after 40 minutes of milling (λ = 0.20735 Å). Red = high intensity, blue = low intensity. Inset: Calculated ZIF-8 PXRD pattern from a literature CIF.20 (b) DSC-TGA scan of amZIF-8 exhibiting a Tx onset = 282 °C. (c) PXRD patterns (Cu-source) of the simulated ZIF-8,20 experimental amZIF-8 and the new phase obtained after heating amZIF-8 to 400 °C. | ||
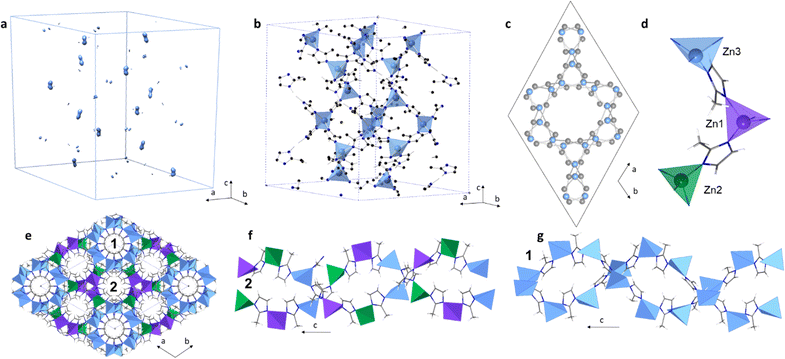
The ball-milling amorphisation step in the synthesis of this new phase meant that the particle size of the crystallised sample was small (<5 μm) and was hence not suitable for single crystal X-ray diffraction (Fig. S5 and S6, ESI†). Therefore, to produce an atomic-scale model of this new phase, structural solution from PXRD data was performed. A high-resolution diffraction pattern was collected on the I11 beamline (Diamond Light Source synchrotron) using X-rays with a wavelength of λ = 0.82697 Å. The pattern was indexed using Materials Studio Suite, with a hexagonal unit cell and with parameters a = 17.3621(8) Å, c = 24.0711(9) Å, V = 6283.926(17) Å3, in the space group P6122. A Pawley refinement based on this cell and space group was performed to obtain integrated intensities (Fobs2) (Table S2 and Fig. S7, ESI†). Electron density maps were produced using the charge flipping method (Fig. 2a and Fig. S8, ESI†).25 Zn positions were identified from the map at special positions with a total of 24 Zn atoms per unit cell (Fig. 2b and Fig. S8, ESI†). C and N atomic positions were refined constraining the mIm linker as a rigid body. The structural model was refined against the diffraction pattern using the Rietveld method (Tables S3 and S9, ESI†). The resulting topology was identified as qtz (Fig. 2c and Fig. S10, ESI†), using TOPOS pro.
According to 1H-NMR and CHN microanalyses, qtz-[Zn(mIm)2] retains the mIm linker and no new proton environments form during its crystallisation (Fig. S11 and Table S4, ESI†). Furthermore, qtz-[Zn(mIm)2] exhibited an almost identical Fourier transform infrared spectrum to both ZIF-8 and amZIF-8, thus indicating the retention of local chemical bonding across all three phases (Fig. S12, ESI†). TGA revealed qtz-[Zn(mIm)2] was thermally stable under argon up to 452 °C, above which it began to decompose rapidly (Fig. S13, ESI†). Multiple heating cycles to 450 °C were performed on qtz-[Zn(mIm)2], though DSC indicated no further phase transitions, suggesting that this crystalline polymorph is thermodynamically stable (Fig. S14, ESI†). This is in agreement with previous studies which identify the qtz topology as one of the most thermodynamically stable crystalline ZIF topologies.26 Surprisingly, the qtz-ZIF-8 material has only been achieved in the presence of the eIm linker, where eIm = 2-ethyl imidazolate (C5H7N2)−.27 In an incredibly recent study, a material exhibiting the qtz topology was prepared by heating a sample of nanosized (20 nm) crystalline SOD-[Zn(mIm)2].28 However, in this case, the authors were not able to obtain cell parameters, or atomic positions within this previously unknown structure. The qtz-[Zn(mIm)2] has some differences to other previously published qtz topology ZIFs, qtz-[M(eIm)2] where M = Zn,29 Fe and Co,27 and [Fe(mIm)2].30 Both qtz-[Fe(mIm)2] and qtz-[M(eIm)2] possess one unique M equivalent position, whilst qtz-[Zn(mIm)2] has three distinct Zn atomic positions each with a distorted tetrahedral environment (Fig. 2d and Fig. S15, Table S5, ESI†). All qtz structures show double helices and channels along the c axis (Fig. S16 and 17, ESI†). qtz-[Zn(mIm)2] has two different channels, where the first one is located along (0, 0, z), and the second one is along (1/2, 1/2, z) and equivalents (Fig. 2e–g). To further confirm that the structure is a new qtz-[Zn(mIm)2] polymorph, a comparison of our experimental PXRD data to the dense dia-[Zn(mIm)2] polymorph is presented in Fig. S18 (ESI†). This clearly highlights the different positions of the Bragg reflections exhibited by the dia and qtz [Zn(mIm)2] structures.
Gas sorption experiments were performed on ZIF-8, amZIF-8, and qtz-[Zn(mIm)2], to investigate their porosity (Fig. S19, ESI†). These showed that qtz-[Zn(mIm)2] has a lower total CO2 uptake than ZIF-8 and amZIF-8; maximum volumes of CO2 adsorbed for ZIF-8, amZIF-8 and qtz-[Zn(mIm)2] were 37.6, 18.6 and 6.5 cm3 g−1 STP, respectively. This is likely because qtz-[Zn(mIm)2] is a denser phase than amZIF-8 and ZIF-8. At higher gas pressures, am[Zn(mIm)2], and especially qtz-[Zn(mIm)2], showed signs of CO2 uptake plateauing, while ZIF-8 remained unsaturated even at the maximum pressure used during these measurements. amZIF-8 shows the largest adsorption–desorption hysteresis due to a kinetic effect caused by the lack of regular pore structure, which restricts CO2 desorption from the material.
To further investigate porosity differences between the three polymorphs of ZIF-8, absorption of methylene blue (MB) dye from an aqueous solution was investigated. MB was chosen as it has been shown to be adsorbed by ZIF-8, where the adsorption mechanism was studied.31 After 48 hours of stirring powdered ZIF samples in a 5 ppm aqueous solution, followed by filtration (see Methods), it was clear on visual inspection that the adsorption of MB by the various ZIF-8 polymorphs followed the trend in porosity seen from CO2 gas absorption experiments, though MB molecules may also be situated in the space between particles (Fig. S20, ESI†). Dye uptake values were determined from UV-VIS absorbance spectra of the remaining solutions at 665 nm by comparing to a calibration curve (Fig. S20, ESI†). The observed trend for MB adsorption was ZIF-8 > amZIF-8 > qtz-[Zn(mIm)2], with absorbance percentages of 92.8%, 80.0% and 43.1% respectively (Fig. S22 and Table. S6, ESI†). This trend might be related with the larger particle size of the qtz-[Zn(mIm)2] phase compared to the small particle size of amZIF-8. These results highlight two important points. Firstly, that amZIFs still possess desirable properties, and even exhibit higher uptake of gases and organic molecules than some crystalline polymorphs, i.e., amZIF-8 > qtz-[Zn(mIm)2]. Secondly, these results show how total gas uptake and crystallinity are not always good predictors of a material's performance in other applications, as qtz-[Zn(mIm)2], and especially amZIF-8, possess far higher dye adsorption than might be first anticipated from their gas uptake properties (Fig. S22, ESI†).
In conclusion, we demonstrate the formation of a new crystalline polymorph of ZIF-8, qtz-[Zn(mIm)2], from mechanically amorphised ZIF-8. This was achieved by heating amZIF-8 under an argon atmosphere. qtz-[Zn(mIm)2] has lower CO2 uptake than amZIF-8 or ZIF-8; most likely due to the high structural density of qtz-[Zn(mIm)2] and its narrow pores. The formation of new crystalline polymorphs obtained by heating the amorphous phases illustrates the complex energetic landscape of ZIF materials and highlights the importance of the amorphous phases of MOFs as metastable intermediates in the formation of new crystalline polymorphs. This study opens up new possibilities for obtaining unprecedented ZIF topologies, even in highly investigated systems such as ZIF-8, by combining mechanochemistry with other energy sources. It also provides firm evidence of the possible utility of amZIFs in water remediation applications.
M. F. T. thanks Corning Incorporated for PhD funding. C. C. B., L. N. M. and T. D. B. acknowledge the Leverhulme Trust for a Research Project Grant (RPG-2020-005), and a Philip Leverhulme Prize (2019). T. D. B., A. M. B. thank the Royal Society (UF150021, RFG\EA\180092, RSG\R1\180395 and RGS\R2\212221). A. F. S. acknowledges the EPSRC under industrial CASE scheme along with Johnson Matthey PLC (JM11106). K. U. thanks the Croatian Science Foundation and the European Social Fund for financial support (PZS-2019-02-4129). We extend our gratitude to Diamond Light Source (United Kingdom), and DESY (Germany) for access to beamlines I11(CY28349-5) and P02.1, respectively.
M. F. T. and C. C. B. conceptualised the project and wrote the manuscript with input of all authors. M. F. T. synthesised and characterised the materials and carried out UV-VIS experiments. L. N. M. collected CO2 isotherms. G. R. collected SEM images. A. M. B., A. F. S. and D. A. K. contributed with useful discussions. C. S. C., F. H. and C. G. collected the PXRD pattern at I-11. M. E., M. F. T. and K. U. collected in situ ball milling XRD experiments. C. C. B. and I. d. S. solved and refined the qtz-[Zn(mIm)2] structure. T. D. B. supervised the project and acquired funding.
Conflicts of interest
There are no conflicts to declare.References
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O’Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- Y.-Q. Tian, C.-X. Cai, Y. Ji, X.-Z. You, S.-M. Peng and G.-H. Lee, Angew. Chem., Int. Ed., 2002, 41, 1384–1386 CrossRef CAS.
- C. Healy, K. M. Patil, B. H. Wilson, L. Hermanspahn, N. C. Harvey-Reid, B. I. Howard, C. Kleinjan, J. Kolien, F. Payet, S. G. Telfer, P. E. Kruger and T. D. Bennett, Coord. Chem. Rev., 2020, 419, 213388 CrossRef CAS.
- B. Chen, Z. Yang, Y. Zhu and Y. Xia, J. Mater. Chem. A, 2014, 2, 16811–16831 RSC.
- R. N. Widmer, G. I. Lampronti, S. Chibani, C. W. Wilson, S. Anzellini, S. Farsang, A. K. Kleppe, N. P. M. Casati, S. G. MacLeod, S. A. T. Redfern, F.-X. Coudert and T. D. Bennett, J. Am. Chem. Soc., 2019, 141, 9330–9337 CrossRef PubMed.
- T. D. Bennett and A. K. Cheetham, Acc. Chem. Res., 2014, 47, 1555–1562 CrossRef CAS.
- M. Stepniewska, K. Januchta, C. Zhou, A. Qiao, M. M. Smedskjaer and Y. Yue, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 10149–10154 CrossRef CAS.
- R. Gaillac, P. Pullumbi, K. A. Beyer, K. W. Chapman, D. A. Keen, T. D. Bennett and F.-X. Coudert, Nat. Mater., 2017, 16, 1149–1154 CrossRef CAS PubMed.
- S. Park and H.-K. Jeong, J. Mater. Chem. A, 2022, 10, 4992–4998 RSC.
- A. M. Bumstead, M. L. Ríos Gómez, M. F. Thorne, A. F. Sapnik, L. Longley, J. M. Tuffnell, D. S. Keeble, D. A. Keen and T. D. Bennett, CrystEngComm, 2020, 22, 3627–3637 RSC.
- R. Gaillac, P. Pullumbi and F. X. Coudert, J. Phys. Chem. C, 2018, 122, 6730–6736 CrossRef CAS.
- E. F. Baxter, T. D. Bennett, A. B. Cairns, N. J. Brownbill, A. L. Goodwin, D. A. Keen, P. A. Chater, F. Blanc and A. K. Cheetham, Dalton Trans., 2016, 45, 4258–4268 RSC.
- T. D. Bennett, P. J. Saines, D. A. Keen, J. C. Tan and A. K. Cheetham, Chem. – Eur. J., 2013, 19, 7049–7055 CrossRef CAS PubMed.
- T. D. Bennett, S. Cao, J. C. Tan, D. A. Keen, E. G. Bithell, P. J. Beldon, T. Friscic and A. K. Cheetham, J. Am. Chem. Soc., 2011, 133, 14546–14549 CrossRef CAS.
- T. D. Bennett, Y. Yue, P. Li, A. Qiao, H. Tao, N. G. Greaves, T. Richards, G. I. Lampronti, S. A. T. Redfern, F. Blanc, O. K. Farha, J. T. Hupp, A. K. Cheetham and D. A. Keen, J. Am. Chem. Soc., 2016, 138, 3484–3492 CrossRef CAS PubMed.
- L. Frentzel-Beyme, M. Kloß, P. Kolodzeiski, R. Pallach and S. Henke, J. Am. Chem. Soc., 2019, 141, 12362–12371 CrossRef CAS PubMed.
- Y. Pan, Y. Liu, G. Zeng, L. Zhao and Z. Lai, Chem. Commun., 2011, 47, 2071–2073 RSC.
- D. Fairen-Jimenez, S. A. Moggach, M. T. Wharmby, P. A. Wright, S. Parsons and T. Düren, J. Am. Chem. Soc., 2011, 133, 8900–8902 CrossRef CAS PubMed.
- H. H. M. Yeung, A. F. Sapnik, F. Massingberd-Mundy, M. W. Gaultois, Y. Wu, D. A. X. Fraser, S. Henke, R. Pallach, N. Heidenreich, O. V. Magdysyuk, N. T. Vo and A. L. Goodwin, Angew. Chem., Int. Ed., 2019, 58, 566–571 CrossRef CAS PubMed.
- C. Mottillo and T. Friščić, Angew. Chem., 2014, 126, 7601–7604 CrossRef.
- S. Cao, T. D. Bennett, D. A. Keen, A. L. Goodwin and A. K. Cheetham, Chem. Commun., 2012, 48, 7805–7807 RSC.
- A. D. Katsenis, A. Puškarić, V. Štrukil, C. Mottillo, P. A. Julien, K. Užarević, M. H. Pham, T. O. Do, S. A. J. Kimber, P. Lazić, O. Magdysyuk, R. E. Dinnebier, I. Halasz and T. Friščić, Nat. Commun., 2015, 6, 1–8 Search PubMed.
- A. M. Bumstead, M. F. Thorne and T. D. Bennett, Faraday Discuss., 2021, 225, 210–225 RSC.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O’Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- F. Gándara, F. J. Uribe-Romo, D. K. Britt, H. Furukawa, L. Lei, R. Cheng, X. Duan, M. O’Keeffe and O. M. Yaghi, Chem. – Eur. J., 2012, 18, 10595–10601 CrossRef PubMed.
- Z. Akimbekov, A. D. Katsenis, G. P. Nagabhushana, G. Ayoub, M. Arhangelskis, A. J. Morris, T. Friščić and A. Navrotsky, J. Am. Chem. Soc., 2017, 139, 7952–7957 CrossRef CAS PubMed.
- J. López-Cabrelles, E. Miguel-Casañ, M. Esteve-Rochina, E. Andres-Garcia, I. J. Vitórica-Yrezábal, J. Calbo and G. Mínguez Espallargas, Chem. Sci., 2022, 13, 842–847 RSC.
- T. Kaneshige, H. Sakamoto and M. Ohtani, Chem. Commun., 2022, 58, 4588–4591 RSC.
- I. Halasz, S. A. J. Kimber, P. J. Beldon, A. M. Belenguer, F. Adams, V. Honkimäki, R. C. Nightingale, R. E. Dinnebier and T. Friščić, Nat. Protoc., 2013, 8, 1718–1729 CrossRef.
- A. L. Spek, A. J. M. Duisenberg and M. C. Feiters, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1983, 39, 1212–1214 CrossRef.
- A. S. Al-Wasidi, I. I. S. AlZahrani, A. M. Naglah, M. G. El-Desouky, M. A. Khalil, A. A. El-Bindary and M. A. El-Bindary, ChemistrySelect, 2021, 6, 11431–11447 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2149756. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc04241j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |