Open Access Article
Open Access ArticlePhotochemical separation of plutonium from uranium†
Ida M.
DiMucci
,
Harrison D.
Root
 ,
Zachary R.
Jones
,
Stosh A.
Kozimor
,
Zachary R.
Jones
,
Stosh A.
Kozimor
 *,
Molly M.
MacInnes
,
Jeffrey L.
Miller
,
Veronika
Mocko
,
Warren J.
Oldham
and
Benjamin W.
Stein
*,
Molly M.
MacInnes
,
Jeffrey L.
Miller
,
Veronika
Mocko
,
Warren J.
Oldham
and
Benjamin W.
Stein
 *
*
Los Alamos National Laboratory, P. O. Box 1663, Los Alamos, NM 87544, USA. E-mail: stosh@lanl.gov; bstein@lanl.gov
First published on 9th September 2022
Abstract
Plutonium-based technologies would benefit if chemical hazards for purifying plutonium were reduced. One critical processing step where improvements could be impactful is the adjustment of plutonium oxidation-states during separations. This transformation often requires addition of redox agents. Unfortunately, many of the redox agents used previously cannot be used today because their properties are deemed incompatible with modern day processing facilities and waste stream safety requirements. We demonstrated herein that photochemistry can be used as an alternative to those chemical agents. We observed that (1) Pu4+ → Pu3+ and UO22+ → U4+ photoreduction proceeded in HCl(aq) and HNO3(aq) and (2) photogenerated Pu3+(aq) and U4+(aq) could be separated using anion exchange chromatography (high yield, >90%; good separation factor, 322).
Many important actinide-technologies rely on chemical processing efforts that generate high purity plutonium and uranium. This spans actinide processing for weapons programs1,2 environmental restoration,3,4 nuclear forensics,5,6 and nuclear fuel reprocessing.7–9 Processing methods that cleanly isolate actinides for these applications often require addition of strong redox agents (oxidants or reductants) that manipulate actinide oxidation states during separations.10 Unfortunately, many effective redox agents used previously have been reclassified as hazardous and cannot be used today. They are now incompatible with modern day nuclear facilities where large quantities of actinides (like Pu) are processed11 or are incompatible with actinide processing waste streams and safe long-term storage in geological repositories. The waste drum breach at the Waste Isolation Pilot Plant in 2014 that dispersed plutonium and americium throughout that facility exemplifies validity of those concerns.12,13 Finding alternative and safe methods to control actinide oxidation states while avoiding addition of harsh chemical agents during processing would have positive impact on actinide-based technologies and waste management activities, alike.
Motivated by this charge, we became intimately aware of recent advances in uranium photochemistry.14 Those studies revealed how uranium oxidation state adjustments could be achieved upon exposure to ultraviolet and visible (UV-vis) light. We were inspired by those discoveries, a limited number of photochemical studies for Pu,15–17 and reports that describe potential for photochemical separation of actinides.18,19 Hence, we set out to develop a photochemical method for Pu/U separations. Our approach was distinct in that it used photochemistry and 2-propanol (a sacrificial electron donor) in place of harsh chemical redox agents to reduce Pu4+(aq) to Pu3+(aq) and UO22+(aq) to U4+(aq) (aq = dissolved in aqueous solutions). This procedure was attractive because it proceeded under aqueous conditions and without oxygen exclusion. It was also rapid (total processing time = 90 min) and could be carried out using commercially available equipment and chemicals (aside from plutonium). Another selling point was that the photoinduced redox events were compatible with ion-exchange chromatography, akin to those used to process large quantities of plutonium from aqueous chloride matrixes, e.g. within the Experimental Chloride Extraction Line (EXCEL) at Los Alamos National Laboratory.20
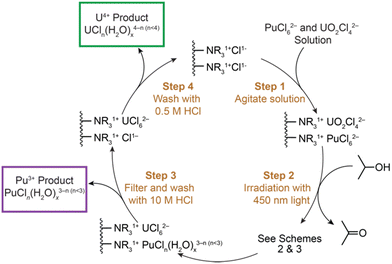
Scheme 1 shows the photochemical separation we developed. It started by dissolving Pu4+(aq) (0.9 mg) (obtained using previously described methods)21 and UO22+(aq) (1.1 mg) in HCl(aq) (10 M, 1% 2-propanol, Fig. 1, brown trace) within a quartz cuvette charged with anion exchange resin (1 mL, AG MP-1, 100 mesh). Combining these reagents caused the brown solution to turn colorless, presumedly because plutonium and uranium adsorbed quantitatively to the resin. This result was consistent with expectations based on the literature. For instance, the Handbook of Ion Exchange Resins22 states both Pu4+(aq) (Kd = >103) and UO22+(aq) (Kd = ∼103) strongly adsorb to anion-exchange resins in HCl(aq) (10 M); Kd is a distribution coefficient, (resin bound analyte/unbound analyte). The next step in the process exposed the colorless mixture to light (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222.2 cm−1; 450 nm). Irradiation at high LED power (100%) rapidly (∼15 min) reduced Pu4+(aq) to Pu3+(aq) and UO22+(aq) to U4+(aq) (vide infra). This oxidation-state adjustment imparted different behavior between plutonium and uranium with the anion exchange resin. It is well established that Pu3+ forms cationic (or neutral) aquo and aquo/chloro complexes [Pu3+Cln(H2O)x3−n; n ≤ 3] in HCl(aq) (10 M) solutions.23–25 These species are not retained by the anion-exchanger's cationic functional groups (NR41+). Hence, the resin released Pu3+(aq) and the mixture turned blue. Next, the mixture was loaded into an empty Bio-Rad column, which filtered the resin from the supernatant. Under these conditions, Pu3+(aq) eluted from the column and the Pu3+ product was collected. Analysis using UV-vis spectroscopy confirmed presence of Pu3+(aq) (Fig. 1, purple trace).21,26
222.2 cm−1; 450 nm). Irradiation at high LED power (100%) rapidly (∼15 min) reduced Pu4+(aq) to Pu3+(aq) and UO22+(aq) to U4+(aq) (vide infra). This oxidation-state adjustment imparted different behavior between plutonium and uranium with the anion exchange resin. It is well established that Pu3+ forms cationic (or neutral) aquo and aquo/chloro complexes [Pu3+Cln(H2O)x3−n; n ≤ 3] in HCl(aq) (10 M) solutions.23–25 These species are not retained by the anion-exchanger's cationic functional groups (NR41+). Hence, the resin released Pu3+(aq) and the mixture turned blue. Next, the mixture was loaded into an empty Bio-Rad column, which filtered the resin from the supernatant. Under these conditions, Pu3+(aq) eluted from the column and the Pu3+ product was collected. Analysis using UV-vis spectroscopy confirmed presence of Pu3+(aq) (Fig. 1, purple trace).21,26
During the Pu3+(aq) elution, U4+(aq) remained retained by the resin. Under these experimental conditions [10 M HCl(aq)], U4+(aq) forms anionic coordination complexes. We naively formulated the U4+ resin-bound species as “UCl62−.” However, lower (UCl51−) and higher (UCl73−) chloride-containing compounds were possible, as were heteroleptic aquo/chloro compounds [UCln(H2O)x4−n, n > 4] and oligomerization products.27 Regardless of identity for the resin bound uranium species, the anion-exchanger's cationic quaternary ammonium functional groups (NR41+) retained the nominal “UCl62−” anion (U4+Kd ≅ 102)22 in HCl(aq) (10 M). After plutonium removal, dilute HCl(aq) (0.5 M) was passed through the resin. This decrease in Cl1−(aq) concentration reduced the number of Cl1− ligands bound to U4+ (Scheme 1), lowered the U4+Kd value (<10),22 and enabled the cationic (or neutral) uranium aquo/chloro [UIVCln(H2O)x4−n; n ≤ 4] species to elute from the column and be cleanly isolated. Analyses using UV-vis spectroscopy confirmed presence of U4+(aq) (Fig. 1, green trace).14,27
The Pu3+(aq) and U4+(aq) products were isolated from this procedure in high yield and in chemically pure forms. Analyses of the Pu3+ product using inductively coupled plasma-mass spectrometry (ICP-MS) and isotope dilution techniques28 showed a 97.5% plutonium recovery yield, 9.2 uranium decontamination factor (Uingoing/UPu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Product), and 322 plutonium/uranium separation factor (SFPu/U); SFPu/U = (PuPu
Product), and 322 plutonium/uranium separation factor (SFPu/U); SFPu/U = (PuPu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Product/PuU
Product/PuU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Product) ÷ (UU
Product) ÷ (UU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Product/UPu
Product/UPu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Product). Similarly positive results were obtained for the U4+ product; 90% uranium yield and 40.6 plutonium decontamination (Puingoing/PuU
Product). Similarly positive results were obtained for the U4+ product; 90% uranium yield and 40.6 plutonium decontamination (Puingoing/PuU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Product). These metrics were comparable to results obtained when using conventional redox agents under equivalent experimental conditions (1 mg of Pu, 1 mg of U, and 1 ml of resin). Larger separation and decontamination factors were obtained when the amounts of plutonium and uranium were decreased, the amount of resin increased, and/or the procedure was repeated multiple times.
Product). These metrics were comparable to results obtained when using conventional redox agents under equivalent experimental conditions (1 mg of Pu, 1 mg of U, and 1 ml of resin). Larger separation and decontamination factors were obtained when the amounts of plutonium and uranium were decreased, the amount of resin increased, and/or the procedure was repeated multiple times.
Studies that generated insight into the redox reactions governing the above plutonium and uranium separations, photochemical reductions, and analyte behaviors with the anion-exchange resin were carried out. These studies utilized pristine stock solutions containing only Pu4+(aq) or UO22+(aq) (vs. the Pu/U mixtures described above) and were carried out within the context of UO22+ photochemistry results published previously in HNO3(aq).29–35 For example, UO22+(aq) photoreduction to U4+(aq) has been shown to proceed with a variety of reductants, including triethylamine,36 formate,35 and 2-propanol.14 We focused this study on 2-propanol, (CH3)2CHOH, as a representative sacrificial electron donor (eqn (1) in Scheme 2). In HNO3(aq), it is proposed that the “photoexcited UO22+(aq)” ion undergoes electron transfer with sacrificial (CH3)2CHOH to generate a UO2˙/+(aq) radical cation alongside the somewhat long-lived organic radical (CH3)2C˙OH.14 The authors provided evidence that successive reactions between these species generate U4+(aq), water (H2O), and acetone [(CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]. Armed with this insight, we reproduced the aforementioned reports and showed that pristine solutions of UO22+(aq) (25 mM) could be photoreduced to U4+(aq) using a mixture of HNO3(aq) (1 M; pH = 0), 2-propanol (1% by volume), and a Penn Photoreactor (22
O]. Armed with this insight, we reproduced the aforementioned reports and showed that pristine solutions of UO22+(aq) (25 mM) could be photoreduced to U4+(aq) using a mixture of HNO3(aq) (1 M; pH = 0), 2-propanol (1% by volume), and a Penn Photoreactor (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222.2 cm−1; 450 nm excitation). We subsequently shifted our focus and demonstrated photoreductions could also occur in HCl(aq) (10 M; pH = −1). This was critical for developing our photochemical separations because UO22+(aq) behaves differently with anion exchange resins in HCl(aq) (retained) vs. HNO3(aq) (not retained).22 We also overserved by NMR spectroscopy that photoreduction of UO22+(aq) to U4+(aq) in HCl(aq) generated (CH3)2C
222.2 cm−1; 450 nm excitation). We subsequently shifted our focus and demonstrated photoreductions could also occur in HCl(aq) (10 M; pH = −1). This was critical for developing our photochemical separations because UO22+(aq) behaves differently with anion exchange resins in HCl(aq) (retained) vs. HNO3(aq) (not retained).22 We also overserved by NMR spectroscopy that photoreduction of UO22+(aq) to U4+(aq) in HCl(aq) generated (CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (see ESI†). Hence, the reaction pathway described in Scheme 2 may appropriately describe photoreduction of UO22+(aq) to U4+(aq) in HCl(aq), as well as in HNO3(aq). Obviously, more data is needed to confirm that proposition.
O (see ESI†). Hence, the reaction pathway described in Scheme 2 may appropriately describe photoreduction of UO22+(aq) to U4+(aq) in HCl(aq), as well as in HNO3(aq). Obviously, more data is needed to confirm that proposition.
To provide insight into uranium speciation during the electron transfer process we monitored the photochemical reduction of UO22+(aq) in HCl(aq)in situ using UV-vis spectroscopy (Fig. 2). The characteristic manifold of UV-vis absorbances from UO22+(aq) in HCl(aq) (10 M) between 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 25
000 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 (500 to 400 nm) was unaffected upon addition of 2-propanol (1% by volume). This data suggested UO22+(aq) had no affinity for alcohol complexation under these conditions. Irradiating this solution at 22
000 cm−1 (500 to 400 nm) was unaffected upon addition of 2-propanol (1% by volume). This data suggested UO22+(aq) had no affinity for alcohol complexation under these conditions. Irradiating this solution at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222 cm−1 (450 nm) changed the yellow color to green, wilted the UO22+(aq) absorbances, and generated peaks characteristic of U4+(aq).37 The spectrum was dominated by a split absorption band between 14
222 cm−1 (450 nm) changed the yellow color to green, wilted the UO22+(aq) absorbances, and generated peaks characteristic of U4+(aq).37 The spectrum was dominated by a split absorption band between 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 286 and 16
286 and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666 cm−1 (700 to 600 nm) with maxima at 14
666 cm−1 (700 to 600 nm) with maxima at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 859 and 15
859 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 385 cm−1 (650 and 673 nm) that were followed at higher energy by a series of three lower intensity absorptions (17
385 cm−1 (650 and 673 nm) that were followed at higher energy by a series of three lower intensity absorptions (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 929, 20
929, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205, and 22
205, and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 729 cm−1).27 Comparisons of UV-vis data pre- and post-irradiation suggested quantitative conversion of UO22+(aq) to U4+(aq) occurred in HCl(aq) (60 min, LED power = 25%). We also report that UO22+ photoreduction did not occur in the absence of 2-propanol. This reagent was clearly critical.
729 cm−1).27 Comparisons of UV-vis data pre- and post-irradiation suggested quantitative conversion of UO22+(aq) to U4+(aq) occurred in HCl(aq) (60 min, LED power = 25%). We also report that UO22+ photoreduction did not occur in the absence of 2-propanol. This reagent was clearly critical.
Although, plutonium photoreduction has been investigated far less than UO22+, one relevant report came from Toth and coworkers in the 1970s.18 These researchers observed photochemical reduction of Pu4+(aq) to Pu3+(aq) in HNO3(aq) (1.2 M) using an irradiation source of 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333 cm−1 (300 nm) and a variety of sacrificial electron donors (formic acid, ethanol, and hydrazine). Emboldened by those results and the UO22+(aq) photochemistry described above, we proposed Pu4+(aq) would undergo a similar reduction in the presence of (CH3)2CHOH in HNO3(aq) and HCl(aq) matrixes (see Scheme 3 and ESI†). The UV-vis data we obtained in HCl(aq) (10 M, Fig. 2) and HNO3(aq) (1 M, see ESI†) were consistent with that hypothesis. Even though we conducted experiments in HNO3(aq) (see ESI†), our focus was on reduction in HCl(aq) because this matrix provided desirable conditions for achieving separations with anion exchange chromatography. Fig. 2 shows the start of the photoreduction (black trace), which represented a solution of Pu4+(aq) dissolved in HCl(aq) (10 M). This spectrum contained the two Pu4+(aq) absorption bands with peak maxima 14
333 cm−1 (300 nm) and a variety of sacrificial electron donors (formic acid, ethanol, and hydrazine). Emboldened by those results and the UO22+(aq) photochemistry described above, we proposed Pu4+(aq) would undergo a similar reduction in the presence of (CH3)2CHOH in HNO3(aq) and HCl(aq) matrixes (see Scheme 3 and ESI†). The UV-vis data we obtained in HCl(aq) (10 M, Fig. 2) and HNO3(aq) (1 M, see ESI†) were consistent with that hypothesis. Even though we conducted experiments in HNO3(aq) (see ESI†), our focus was on reduction in HCl(aq) because this matrix provided desirable conditions for achieving separations with anion exchange chromatography. Fig. 2 shows the start of the photoreduction (black trace), which represented a solution of Pu4+(aq) dissolved in HCl(aq) (10 M). This spectrum contained the two Pu4+(aq) absorption bands with peak maxima 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 cm−1 (701 nm) and 18
260 cm−1 (701 nm) and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 005 cm−1 (555 nm). The spectrum was unaffected by addition of 2-propanol, again suggesting that no complexation of 2-propanol by Pu4+(aq) occurred under these conditions. Substantial changes were detected, however, when the solution was irradiated (22
005 cm−1 (555 nm). The spectrum was unaffected by addition of 2-propanol, again suggesting that no complexation of 2-propanol by Pu4+(aq) occurred under these conditions. Substantial changes were detected, however, when the solution was irradiated (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222 cm−1, 450 nm). The Pu4+(aq) absorption bands waned amongst emerging peaks from Pu3+(aq) (16
222 cm−1, 450 nm). The Pu4+(aq) absorption bands waned amongst emerging peaks from Pu3+(aq) (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 639 and 17
639 and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 857 cm−1; 560 and 601 nm). UV-Vis assays showed quantitative conversion of Pu4+(aq) to Pu3+(aq) within 60 min (LED power = 25%; Fig. 1 and 2, purple trace). The formation of (CH3)2C
857 cm−1; 560 and 601 nm). UV-Vis assays showed quantitative conversion of Pu4+(aq) to Pu3+(aq) within 60 min (LED power = 25%; Fig. 1 and 2, purple trace). The formation of (CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was confirmed by 1H NMR spectroscopy, and the transformation is described in eqn (4) and (5). Again, 2-proponal was essential for the photoreduction.
O was confirmed by 1H NMR spectroscopy, and the transformation is described in eqn (4) and (5). Again, 2-proponal was essential for the photoreduction.
We have demonstrated that Pu4+(aq) and UO22+(aq) undergo photoreduction to Pu3+(aq) and U4+(aq) in HCl(aq) in the presence of 2-propanol. We further showed that this oxidation state adjustment was compatible with ion-exchange chromatography and developed a photochemical separation of plutonium from uranium. Our separation was effective, efficient, and generated chemically pure U4+(aq) and Pu3+(aq) products in high yield. It could be carried out rapidly using a commercially available photoreactor in aqueous media without exclusion of air. Our usage of photochemistry in place of harsh redox agents during actinide separations was attractive on many other levels. First and foremost, the substitution keeps harsh (i.e. hydroxylamine, sodium nitrite, sodium chlorite) redox agents out of process waste streams. This reduces threat of waste container failure promoted by unwanted side-reactions and pressurization that can occur during long-term storage. Photoreduction can additionally limit spread of contamination during oxidation-state adjustments. For example, bubbling and splattering (sometime vigorously) can occur when common reducing agents (e.g. hydroxylamine hydrochloride) are added to actinide containing acidic solutions.21 This hazard is avoided by photochemical reduction. The substitution also avoids corrosive redox agents (e.g. bromine containing chemicals)38 known to deteriorate critical hardware in processing facilities (like radiological gloveboxes and hoods). We are excited to implement this photochemical separation method locally when we recover and reprocess small quantities of plutonium for our research campaigns. Potential application also resides outside of our laboratory, within the realm of actinide analytical chemistry (for environmental monitoring and nuclear forensics1–9) and for large-scale actinide processing (like EXCEL).20 From this perspective, data reported herein provides an intriguing “proof-of-principle.” We hope these results inspire others to explore photochemical actinide separations, especially as a tool to improve the safety, efficiency, and effectiveness of actinide processing efforts.
We thank the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Heavy Element Chemistry program (2020LANLE372) and LANL's Laboratory Directed Research and Development program (20190364ER). Postdoctoral support was provided in part by the Los Alamos National Laboratory Named Agnew (DiMucci) and the Glenn T. Seaborg Institute (Root, Jones) Post-Doctoral Fellowships. LANL is an affirmative action/equal opportunity employer managed by Triad National Security, LLC, for the National Nuclear Security Administration of the U.S. DOE.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- A. B. Lovins, Nature, 1980, 283, 817–823 CrossRef CAS.
- D. E. Hunter, R. T. Hutton, M. Breen, P. F. Bronson, W. A. Chambers, G. A. Davis, D. S. Disraelly, P. A. M. Dolph, D. R. Gillingham, J. M. Gilmore, D. R. Graham, D. E. Lago, T. K. Mitchell, E. M. Parrish, M. J. Reed, D. M. Tate, J. K. Warden and K. Y. Wu, 2019. IDA Document NS D-10711 H 10-000304.
- W. H. E. Schwarz, N. M. Shavaleev, S. N. Kalmykov, A. Y. Romanchuk and I. E. Vlasova, Front. Chem., 2020, 1, 630–640 Search PubMed.
- H. Geckeis, B. Salbu, T. Schafer and M. Zavarin, 2019. http://www.osti.gov/servlets/purl/1606477.
- M. J. Kristo, 2018. http://www.osti.gov/servlets/purl/1603873.
- Z. Varga, M. Wallenius, M. Krachler, N. Rauff-Nisthar, L. Fongaro, A. Knott, A. Nicholl and K. Mayer, Trends Anal. Chem., 2022, 146, 116503–116512 CrossRef CAS.
- D. D. Sood and S. K. J. Patil, J. Radioanal. Nucl. Chem., 1996, 203(2), 547–573 CrossRef CAS.
- M. F. Simpson, J. D. Law, INL/EXT-10-17753 Report, 2013, DOI:10.2172/974763.
- J. E. Birkett, M. J. Carrott, O. Danny, C. J. Jones, C. J. Maher, C. V. Roube, R. J. Taylor and D. A. Woodhead, J. Nucl. Sci. Technol., 2007, 44(3), 337–343 CrossRef CAS.
- P. Baron, S. M. Cornet, E. D. Collins, G. DeAngelis, G. Del Cul, Y. Fedorov, J. P. Glatz, V. Ignatiev, T. Inoue, A. Khaperskaya, I. T. Kim, M. Kormilitsyn, T. Koyama, J. D. Law, H. S. Lee, K. Minato, Y. Morita, J. Uhlíř, D. Warin and R. J. Taylor, Prog. Nucl. Energy, 2019, 117, 103091–103115 CrossRef CAS.
- P. Paviet-Hartmann, C. Riddle, K. Campbell and E. Mausolf, 2013. https://inldigitallibrary.inl.gov/sites/sti/sti/6037803.pdf.
- A. J. Schramke, E. F. U. Santillan and R. T. Peake, Appl. Geochem., 2020, 116, 104561–104576 CrossRef PubMed.
- US Department of Energy. Accident Investigation Report: Phase 2, Radiological Release Event at the Waste Isolation Pilot Plant, February 14, 2014, 2015.
- M. C. Rath, S. J. Keny and D. B. Naik, J. Photochem. Photobiol., A, 2019, 376, 155–159 CrossRef CAS.
- S. Sasaki, Y. Wada and H. Tomiyasu, Prog. Nucl. Energy, 1998, 32(3–4), 403–410 CrossRef CAS.
- J. T. Bell and H. A. J. Friedman, J. Inorg. Nucl. Chem., 1976, 38(4), 831–835 CrossRef CAS.
- M. Goldstein, J. J. Barker and T. Gangwer, 1976, http://www.osti.gov/servlets/purl/7294803.
- L. M. Toth, H. A. Friedman and J. T. Bell, 1977, https://www.osti.gov/biblio/7102579.
- L. M. Toth, J. T. Bell and H. A. Friedman, 1979, https://www.osti.gov/servlets/purl/6064818.
- D. L. Clark, G. D. Jarvinen, C. Kowalczyk, J. Rubin and M. A. Stroud, Actinide Research Quarterly: Plutonium Processing at Los Alamos, 2008, https://www.lanl.gov/discover/publications/actinide-research-quarterly/pdfs/ARQ-2008-10.pdf.
- S. K. Cary, K. S. Boland, J. N. Cross, S. A. Kozimor and B. L. Scott, Polyhedron, 2017, 126, 220–226 CrossRef CAS.
- J. Korkisch, CRC Handbook of Ion Exchange Resins, CRC Press, 2017, vol. VI Search PubMed.
- D. L. Clark, Plutonium Handbook, American Nuclear Society, 2nd edn, 2019 Search PubMed.
- P. G. Allen, J. J. Bucher, D. K. Shuh, N. M. Edelstein and T. Reich, Inorg. Chem., 1997, 36(21), 4676–4683 CrossRef CAS.
- C. Henning, J. Tutschku, A. Rossberg, G. Bernhard and A. C. Scheinost, Inorg. Chem., 2005, 44(19), 6655–6661 CrossRef.
- W. T. J. Carnall, Chem. Phys., 1992, 96(12), 8713–8726 CAS.
- J. Tutschku, C. Hennig and G. G. Bernhard, FZR-IRC Annual Report, 2004, p. 12 Search PubMed.
- A. Gaffney, Guideline on Isotope Dilution Mass Spectrometry, 2017, https:/doi.org/10.2172/1358328 Search PubMed.
- V. N. Salomone, J. M. Meichtry, G. Schinelli, A. G. Leyva and M. I. J. Litter, J. Photochem. Photobiol., A, 2014, 277, 19–26 CrossRef CAS.
- S. Tsushima, Inorg. Chem., 2009, 48(11), 4856–4862 CrossRef CAS PubMed.
- M. Kobylarski, L. Monsigny, P. Thuéry, J. C. Berthet and T. Cantat, Inorg. Chem., 2021, 60(21), 16140–16148 CrossRef CAS PubMed.
- R. Nagaishi, Y. Katsumura, K. Ishigure, H. Aoyagi, Z. Yoshida and T. Kimura, J. Photochem. Photobiol., A, 1996, 96, 45–50 CrossRef CAS.
- P. Li, J. Wang, Y. Wang, J. Liang, B. He, D. Pan, Q. Fan and X. Wang, Chem. Eng. J., 2019, 365, 231–241 CrossRef CAS.
- M. C. Rath, S. Keny and D. B. J. Naik, Nanosci. Nanotechnol., 2016, 16(9), 9575–9582 CAS.
- T. M. Mccleskey, T. M. Foreman, E. E. Hallman, C. J. Burns and N. N. Sauer, Environ. Sci. Technol., 2001, 35, 547–551 CrossRef CAS.
- M. S. Sidhu, K. B. Kohli, P. V. K. Bhatia and S. S. Sandhu, J. Radioanal. Nucl. Chem. Lett., 1994, 187(5), 375–383 CrossRef CAS.
- E. Hashem, A. N. Swinburne, C. Schulzke, R. C. Evans, J. A. Platts, A. Kerridge, L. S. Natrajan and R. J. Baker, RSC Adv., 2013, 3, 4350 RSC.
- S. F. Marsh, 1980. https://www.osti.gov/servlets/purl/5051957.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc04225h |
| This journal is © The Royal Society of Chemistry 2022 |