A three-component difunctionalization of N-alkenyl amides via organophotoredox radical-polar crossover†
Abstract
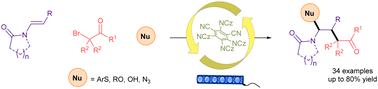
Herein, we report a three-component organophotoredox coupling of N-alkenyl amides with α-bromocarbonyls and various nucleophiles. This transition metal-free difunctionalization protocol installs sequential C–C and C–Y (Y = S/O/N) bonds in alkenes. This reaction works with terminal and internal alkenes containing both cyclic and acyclic amides via radical-polar crossover.


 Please wait while we load your content...
Please wait while we load your content...