Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Air-stable aryl derivatives of pentafluoroorthotellurate†
Daniel
Wegener
 ,
Kurt F.
Hoffmann
,
Kurt F.
Hoffmann
 ,
Alberto
Pérez-Bitrián
,
Alberto
Pérez-Bitrián
 ,
Ilayda
Bayindir
,
Amiera N.
Hadi
,
Anja
Wiesner
and
Sebastian
Riedel
,
Ilayda
Bayindir
,
Amiera N.
Hadi
,
Anja
Wiesner
and
Sebastian
Riedel
 *
*
Fachbereich Biologie, Chemie, Pharmazie, Institut für Chemie und Biochemie – Anorganische Chemie, Freie Universität Berlin, Fabeckstraße 34/36, 14195 Berlin, Germany. E-mail: s.riedel@fu-berlin.de
First published on 8th August 2022
Abstract
We report on two different sets of air-stable derivatives of pentafluoroorthotellurate containing fluorinated and non-fluorinated aryl groups. The acid cis-PhTeF4OH was obtained in gram scale and further transformed to Ag[cis-PhTeF4O], which was used as a cis-PhTeF4O transfer reagent to obtain [PPh4][cis-PhTeF4O]. Furthermore, the synthesis of trans-(C6F5)2TeF3OH was achieved by a selective hydrolysis of trans-(C6F5)2TeF4 in the presence of KF and subsequent protonation by aHF. Quantum-chemical calculations show a higher acidity and robustness against fluoride abstraction for trans-(C6F5)2TeF3OH compared to cis-PhTeF4OH.
The pentafluoroorthotellurate group (teflate, OTeF5) is known for its robustness against oxidizers and electrophiles, the high delocalization of the negative charge, and its strong electron withdrawing properties, which are comparable to fluorine.1,2 As a consequence, the teflate group provides access to a variety of weakly coordinating anions (WCAs),3–5 strong Lewis acids5 and highly reactive species.1–3,6 Nevertheless, compounds containing teflate groups also present some drawbacks, which include the sensitivity to hydrolysis leading to the formation of degradation products (e.g. HF).1 Additionally, the synthesis of HOTeF5, which is the most commonly used teflate source, requires special starting materials (Te(OH)6 and HSO3F).7,8 These properties severely limit the potential field of application of the OTeF5 group and only allow the work under strictly inert conditions.
During the last years (perfluoro)organotellurium chemistry has expanded significantly.9,10 The substitution of some of the fluorine atoms in the OTeF5 group by (perfluoro)aryl groups might help overcome its instability towards water.11 The access to organotellurium(VI) fluorides has been historically achieved through the oxidative fluorination of organotellurium compounds in lower oxidation states. However, this required the use of harsh conditions and strong oxidizers, leading in most cases to low yields or impure reaction mixtures, which could not be always isolated.12–14 For example, the oxidation of Te(C6F5)2 with elemental fluorine led to (C6F5)2TeF2 and cis-(C6F5)2TeF4 stepwise, but faced the problem of further fluorination of the aryl rings.12 On the other hand, PhTeF5 was prepared via oxidation of Ph2Te2 with XeF2 and, although it was of great interest for reactivity studies,15–17 it was never isolated prior to use.13–17
Recently, a new system for the oxidative fluorination of diarylditellurides consisting of trichloroisocyanuric acid (TCICA), potassium fluoride and catalytic amounts of trifluoroacetic acid has been reported.18 This system has been successfully applied to the synthesis of a broad scope of TeF5-substituted arenes in good yields, which include the PhTeF5 derivative. The easy access to these compounds has therefore allowed the study of the properties and reactivity of the TeF5 moiety.11,18,19 Compound PhTeF5 was found to be an air-stable molecule, in contrast to previous descriptions, yet could be hydrolysed quantitatively to cis-PhTeF4OH in a mixture of acetonitrile and water.18
Herein we report on the synthesis and characterization of two different aryl-substituted derivatives of the pentafluoroorthotellurate, namely [cis-PhTeF4O]− and [trans-(C6F5)2TeF3O]−,20 and their corresponding Brønsted acids.
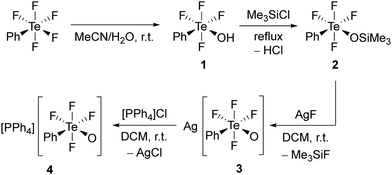
Compound cis-PhTeF4OH (1) was prepared through a modified procedure (see ESI† for details).18 It was obtained as a colourless oil in gram scale and in excellent yield (Scheme 1). The cis arrangement of the phenyl and hydroxy substituents is observed both in solution and in the solid state, as confirmed by 19F NMR spectroscopy and single-crystal X-ray diffraction, respectively. Compound 1 represents the first crystallized species containing the TeF4OH moiety connected to an aryl and can be considered as a derivative of HOTeF5. The Brønsted acid cis-PhTeF4OH crystallizes in the orthorhombic space group Pbca and shows a distorted octahedral arrangement at the Te centre (Fig. 1), with C–Te–O angles of 93.9(1)° and 98.6(1)° and Te–F bonds between 184.8(2) and 187.4(2) pm. Two independent molecules are found in the asymmetric unit, which are connected through a hydrogen bond. The O⋯O distance is 270.7 pm, therefore being in the expected range for such non-covalent interaction.21
 | ||
| Fig. 1 Molecular structure of cis-PhTeF4OH (1) in the solid state. Displacement ellipsoids set at 50% probability. Selected bond lengths [pm] and angles [°]: Te1–F1 187.3(2), Te1–F2 185.5(2), Te1–F3 186.6(2), Te1–F4 187.0(2), Te1–O1 189.7(2), Te1–C1 207.2(3), O1–Te1–F1 172.0(1), C1–Te1–F2 178.2(1), F3–Te1–F4 170.5(1), C1–Te1–O1 93.9(1), Te2–F5 187.4(2), Te2–F6 184.8(2), Te2–F7 186.9(2), Te2–F8 186.4(2), Te2–O2 189.7(2), Te2–C7 207.8(3), O1···O2 270.7, O2–Te2–F5 167.8(1), C7–Te2–F6 178.1(1), F7–Te2–F8 170.4(1), C7–Te2–O2 98.6(1). For crystallographic details see ESI.† | ||
The 19F NMR spectrum of 1 shows the characteristic ABC2X spin system with signals at −25.8, −47.0, and −50.5 ppm and corresponding 2J(19F,19F) coupling constants of 148, 134, and 109 Hz. The NMR spectroscopic data are in agreement with those previously reported by Togni et al.,18 and are also comparable with the signals reported for cis-PhTeF4Cl.22
Contrary to the synthesis of [N(n-Bu)4][OTeF5], which is readily prepared from HOTeF5 and [N(n-Bu)4]Cl,8 compound 1 does not react with a comparable chloride salt such as [PPh4]Cl. In order to increase the reactivity and achieve the transfer of a cis-PhTeF4O group, we aimed at preparing the silver salt of the [cis-PhTeF4O]− anion and using the formation of poorly soluble silver halides as the driving force of the corresponding reaction. With this objective, cis-PhTeF4OH (1) was reacted with Me3SiCl to form cis-PhTeF4OSiMe3 (2), which could be subsequently transformed into Ag[cis-PhTeF4O] (3) selectively (see Scheme 1). The preparation of cis-PhTeF4OSiMe3 (2) from cis-PhTeF4OH (1) was carried out according to a literature procedure reported for related compounds.23
The transformation of cis-PhTeF4OSiMe3 (2) into Ag[cis-PhTeF4O] (3) was achieved by reaction with AgF in dichloromethane, whereby the stable and volatile Me3SiF was formed. The reaction reaches full conversion after 16 h at room temperature. The 19F NMR spectra of 2 and 3 show similar patterns in agreement with the ABC2X spin system. The salt Ag[cis-PhTeF4O] (3) is poorly soluble in many organic solvents, but moderately in acetonitrile, although the solubility increases significantly by the addition of pyridine.
Compound 3 is a suitable transfer reagent of the air-stable cis-PhTeF4O group, as it was shown by its reaction with [PPh4]Cl in dichloromethane. The resulting salt [PPh4][cis-PhTeF4O] (4) was isolated as a colourless solid in 92% yield after filtration to separate the formed AgCl. Single crystals of [PPh4][cis-PhTeF4O] grew in the monoclinic space group P21/c. The molecular structure in the solid state represents the first example of a structurally characterized [RTeF4O]− anion (R = aryl). The [cis-PhTeF4O]− anion possesses a distorted octahedral arrangement at the Te centre (Fig. 2) with marginally longer Te–F (189.3(1)–190.7(1) pm) and Te–O (179.8(2) pm) bonds than in the teflate anion (Te–F: 184.6–186.2 pm; Te–O: 178.9 pm).24 The 19F NMR spectrum shows the previously mentioned typical pattern for such derivatives.
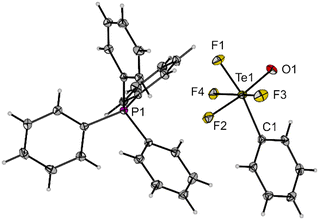 | ||
| Fig. 2 Molecular structure of [PPh4][cis-PhTeF4O] (4) in the solid state. Displacement ellipsoids set at 50% probability. Selected bond lengths [pm] and angles [°]: Te1–F1 189.3(1), Te1–F2 190.5(1), Te1–F3 190.1(1), Te1–F4 190.7(1), Te1–O1 179.8(2), Te1–C1 210.5(2), O1–Te1–F2 173.7(1), C1–Te1–F1 167.4(1), F3–Te1–F4 165.4(1), C1–Te1–O1 99.1(1). For crystallographic details see ESI.† | ||
To determine the robustness against fluoride abstraction of the [cis-PhTeF4O]− anion in the context of different teflate derivatives, the fluoride ion affinities (FIA) of the corresponding neutral oxo compounds have been calculated (see Table 1). As expected, fluorination of the aryl group increases the fluoride ion affinity (FIA), therefore meaning that the [cis-(C6F5)TeF4O]− and [trans-(C6F5)2TeF3O]− anions possess Te–F bonds that are less prone to fluoride abstraction. Additionally, the proton affinities of the corresponding bases also increase upon fluorination of the aryl groups (see Table 1), which shows that the corresponding Brønsted acid is more suitable for protonation reactions aiming at the transfer of the teflate derivative. Both trends prompted us to develop a synthetic route to access a derivative containing C6F5 groups.
| System | FIAb/kJ mol−1 | PAc/kJ mol−1 |
|---|---|---|
| a Trans refers to the relative arrangement of the aryl rings (cf.5). b DFT calculations performed on BP86/def-SV(P) level of theory. Isodesmic reactions with Me3SiF/Me3Si+ as anchor were used.25 c DFT calculations performed on B3LYP/def2-TZVPP level of theory. | ||
| OTeF4/[OTeF5]− | 454 | 1281 |
| PhTeF3O/[cis-PhTeF4O]− | 349 | 1352 |
| Ph2TeF2O/[trans-Ph2TeF3O]− | 294 | 1388 |
| (C6F5)TeF3O/[cis-(C6F5)TeF4O]− | 407 | 1302 |
| (C6F5)2TeF2O/[trans-(C6F5)2TeF3O]− | 368 | 1308 |
The aforementioned TCICA/KF oxidation system was applied to the fluorination of Te(C6F5)2, leading to the isolation of trans-(C6F5)2TeF4 (5) as a white solid in 85% yield (Scheme 2). In contrast, fluorination with elemental fluorine at low temperature leads to cis-(C6F5)2TeF4 quantitatively, as described by Naumann et al. in 1985.12 Our reaction represents the first application of the TCICA/KF oxidation system to a telluride with two perfluorinated substituents, although the use of this protocol with diorganyl monotellurides has been preliminary demonstrated.18,26 More interestingly, it shows the tolerance of the system to compounds with ortho-substituted aromatics, in contrast to previous assumptions.18
The trans arrangement of the two C6F5 rings at the Te centre is easily assigned from the 19F NMR spectrum, as only one resonance for the equatorial fluorine atoms is observed. It appears as a quintet at a chemical shift of −21.4 ppm, with coupling constants of 4J(19F,19Fo) = 19 Hz and 1J(19F,125Te) = 3104 Hz. The 125Te NMR spectrum shows a quintet of multiplets at 770 ppm due to coupling of the tellurium to the four directly bound equatorial F, as well as to the fluorine atoms of the aromatic rings.
Single crystals of 5 suitable for X-ray diffraction were grown from a n-hexane solution of the compound at −40 °C. The compound crystallizes in the orthorhombic space group Pbca and the molecular structure in the solid state shows the same trans arrangement of the C6F5 groups as in solution (Fig. 3). The molecule contains an octahedrally coordinated Te centre with Te–F bond lengths (186.4(1) and 187.2(1) pm) similar to 1 (cf.Fig. 1). Compound 5 is a moisture- and air-stable compound, rather unreactive and soluble in different organic solvents (e.g. n-hexane, acetonitrile, DCM, THF, o-DFB), which makes it an excellent precursor for the synthesis of a teflate derivative with improved properties.
 | ||
| Fig. 3 Molecular structure of trans-(C6F5)2TeF4 (5) in the solid state. Displacement ellipsoids set at 50% probability. Selected bond lengths [pm] and angles [°]: Te1–F1 187.2(1), Te1–F2 186.4(1), Te1–C1 208.7(2), C1–Te1–F1 89.7(1), C1–Te1–F2 90.3(1), F1–Te1–F2 89.4(1). For crystallographic details see ESI.† | ||
Due to the equivalent nature of the four Te–F bonds in trans-(C6F5)2TeF4 (5), the selective hydrolysis of only one of them might be challenging. In fact, by using the same hydrolysis protocol as for PhTeF5, no reaction was observed and, unfortunately, heating the reaction mixture to 50 °C led to mixtures containing the doubly hydrolysed species (see ESI†). The functionalization of one Te–F bond of 5 by using Me3SiOMe or Me3SiNMe2, similarly to Janzen et al.,17 was also tested, yet no reaction was observed either. Gratifyingly, we found that the hydrolysis of the second Te–F bond can be prevented if KF is added to the water/acetonitrile mixture. Following this protocol, K[trans-(C6F5)2TeF3O] (6) was obtained in excellent yield (see Scheme 2). The hydrolysis of 5 proceeds with retention of the trans arrangement of the C6F5 substituents. This is observed in the 19F NMR spectrum of 6, which shows a triplet of quintets (δ = 32.3 ppm) and a doublet of quintets (δ = −18.8 ppm), with an integral ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, accounting for the two chemically inequivalent 19F nuclei (2J(19F,19F) = 104 Hz). Additional splitting is present due to the coupling to the Fo of the C6F5 rings (4J(19F,19Fo) ≈ 20 Hz).
2, accounting for the two chemically inequivalent 19F nuclei (2J(19F,19F) = 104 Hz). Additional splitting is present due to the coupling to the Fo of the C6F5 rings (4J(19F,19Fo) ≈ 20 Hz).
Single crystals of K[trans-(C6F5)2TeF3O]·MeCN were obtained by slow diffusion of diethyl ether into an acetonitrile solution of 6 and were examined by X-ray diffraction. The salt crystallizes in the monoclinic space group P21/c and the Te centre in the [trans-(C6F5)2TeF3O]− anion adopts a distorted octahedral geometry with the expected trans arrangement of the C6F5 units (Fig. 4). The C1–Te1–C7 angle is 166.3(1)° and thus, deviates significantly from the ideal angle of 180°. The Te–F (189.8(1)–191.2(1) pm) and Te–O bonds (178.0(1) pm) have approximately the same length as in the [cis-PhTeF4O]− anion (cf.Fig. 2). The molecular structure in the solid state shows a K⋯O interaction with a distance of 258.8(1) pm. Furthermore, the potassium cation is coordinated by one acetonitrile molecule. The salt K[trans-(C6F5)2TeF3O] (6) represents a moisture- and air-stable compound, which shows high solubility in different organic solvents (e.g. toluene, acetonitrile, THF, o-DFB).
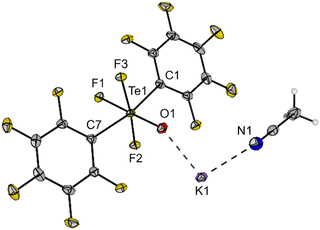 | ||
| Fig. 4 Molecular structure of K[trans-(C6F5)2TeF3O] (6) in the solid state. Displacement ellipsoids set at 50% probability. Selected bond lengths [pm] and angles [°]: Te1–F1 191.1(1), Te1–F2 191.2(1), Te1–F3 189.8(1), Te1–O1 178.0(1), Te1–C1 213.5(2), Te1–C7 212.4(2), K1–O1 258.8(1), O1–Te1–F1 178.9(1), F2–Te1–F3 170.7(1), C1–Te1–C7 166.3(1). For crystallographic details see ESI.† | ||
The need for a fluoride source during the hydrolysis of 5 led to the formation of the anionic [trans-(C6F5)2TeF3O]−, instead of the corresponding acid, in contrast to the synthesis of 1. However, when 6 is treated with aHF, quantitative formation of trans-(C6F5)2TeF3OH (7) takes place. The acid 7 was isolated after extraction in dichloromethane in 77% yield as a hygroscopic solid. In the 19F NMR spectrum, the triplet of quintets is high-field shifted by approx. 30 ppm, compared to the corresponding signal in the 19F NMR spectrum of K[trans-(C6F5)2TeF3O] (6). Moreover, the coupling constant between the fluorine atoms directly bound to the tellurium centre decreases to 2J(19F,19F) = 54 Hz. The protonation becomes also clear from the 1H NMR spectrum, which shows the resonance of the proton as a broad singlet at 5.76 ppm. The IR spectrum of the compound also shows a broad band at 3493 cm−1, which is due to the O–H stretching vibration. Furthermore, in the ESI mass spectrum the signal of the dimer [((C6F5)2TeF3O)2H]− can be observed at m/z = 1070.7.
In conclusion, we developed the synthesis of two different sets of derivatives of the pentafluoroorthotellurate containing aryl substituents. The Brønsted acid cis-PhTeF4OH (1) could be obtained in gram scale and was transformed into the corresponding silver salt Ag[cis-PhTeF4O] (3), which is a suitable transfer reagent of the cis-PhTeF4O group. Its reactivity was demonstrated by the synthesis of [PPh4][cis-PhTeF4O] (4) starting from [PPh4]Cl. Furthermore, we showed the facile synthesis of trans-(C6F5)2TeF4 (5) and its hydrolysis in the presence of KF to form K[trans-(C6F5)2TeF3O] (6), which could be subsequently protonated with aHF to yield the Brønsted acid trans-(C6F5)2TeF3OH (7). Additionally, calculations showed that fluorination of the phenyl rings leads not only to a higher robustness towards fluoride abstraction, but also increases the strength of the corresponding Brønsted acids. Therefore, these compounds are moisture- and air-stable analogues of the teflate, which can be easily obtained with cheap starting materials and through simple procedures. Due to these outstanding properties, both cis-PhTeF4O and trans-(C6F5)2TeF3O arise as promising groups for the synthesis of a new family of WCAs and Lewis acids.
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 387284271—SFB 1349 and the ERC Project HighPotOx. Computing time was made available by the High-Performance Computing Center at the ZEDAT, Freie Universität Berlin. We gratefully acknowledge the assistance of the Core Facility BioSupraMol supported by the DFG. A. P.-B. thanks the Alexander von Humboldt Foundation for a postdoctoral research fellowship.
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Seppelt, Angew. Chem., Int. Ed. Engl., 1982, 21, 877 CrossRef.
- M. Gerken, H. P. A. Mercier and G. J. Schrobilgen, in Advanced Inorganic Fluorides, ed. T. Nakajima, B. Zemva, A. Tressaud, Elsevier, Lausanne, 2000, pp. 117–174 Search PubMed.
- T. A. Engesser, M. R. Lichtenthaler, M. Schleep and I. Krossing, Chem. Soc. Rev., 2016, 45, 789 RSC.
- I. M. Riddlestone, A. Kraft, J. Schaefer and I. Krossing, Angew. Chem., Int. Ed., 2018, 57, 13982 CrossRef CAS PubMed.
- A. Wiesner, T. W. Gries, S. Steinhauer, H. Beckers and S. Riedel, Angew. Chem., Int. Ed., 2017, 56, 8263 CrossRef CAS PubMed.
- H. P. A. Mercier, M. D. Moran, J. C. P. Sanders, G. J. Schrobilgen and R. J. Suontamo, Inorg. Chem., 2005, 44, 49 CrossRef CAS PubMed.
- F. Sladky and C. J. Schack, in Inorganic Syntheses, ed. J. M. Shreeve, John Wiley & Sons, Hoboken, NJ, 1986, pp. 33–37 Search PubMed.
- S. H. Strauss, K. D. Abney and O. P. Anderson, Inorg. Chem., 1986, 25, 2806 CrossRef CAS.
- K. Grollier, A. Taponard and T. Billard, Eur. J. Org. Chem., 2020, 6943 CrossRef CAS.
- T. Chivers and R. S. Laitinen, Chem. Soc. Rev., 2015, 44, 1725 RSC.
- Y. Kraemer, E. N. Bergman, A. Togni and C. R. Pitts, Angew. Chem., Int. Ed., 2022, e202205088 CAS.
- G. Klein and D. Naumann, J. Fluorine Chem., 1985, 30, 259 CrossRef CAS.
- K. Alam and A. F. Janzen, J. Fluorine Chem., 1985, 27, 467 CrossRef CAS.
- X. Ou and A. F. Janzen, J. Fluorine Chem., 2000, 101, 279 CrossRef CAS.
- S. A. Lermontov, S. I. Zavorin, I. V. Bakhtin, A. N. Pushin, N. S. Zefirov and P. J. Stang, J. Fluorine Chem., 1998, 87, 75 CrossRef CAS.
- S. A. Lermontov, S. I. Zavorin, I. V. Bakhtin, N. S. Zefirov and P. J. Stang, Phosphorus, Sulfur Silicon Relat. Elem., 1995, 102, 283 CrossRef CAS.
- A. F. Janzen, K. Alam and B. J. Blackburn, J. Fluorine Chem., 1989, 42, 173 CrossRef CAS.
- D. Bornemann, C. R. Pitts, C. J. Ziegler, E. Pietrasiak, N. Trapp, S. Küng, N. Santschi and A. Togni, Angew. Chem., Int. Ed., 2019, 58, 12604 CrossRef CAS PubMed.
- A. N. Ragan, Y. Kraemer, W.-Y. Kong, S. Prasad, D. J. Tantillo and C. R. Pitts, Angew. Chem., Int. Ed., 2022 DOI:10.1002/anie.202208046.
- In this work, trans refers to the relative arrangement of the aryl rings, in order to keep the stereochemistry as in the precursor 5.
- T. Steiner, Angew. Chem., Int. Ed., 2002, 41, 48 CrossRef CAS.
- X. Ou, G. M. Bernard and A. F. Janzen, Can. J. Chem., 1997, 75, 1878 CrossRef CAS.
- B. Pötter, D. Lentz, H. Pritzkow and K. Seppelt, Angew. Chem., Int. Ed. Engl., 1981, 20, 1036 CrossRef.
- P. K. Miller, K. D. Abney, A. K. Rappe, O. P. Anderson and S. H. Strauss, Inorg. Chem., 1988, 27, 2255 CrossRef CAS.
- H. Böhrer, N. Trapp, D. Himmel, M. Schleep and I. Krossing, Dalton Trans., 2015, 44, 7489 RSC.
- D. Bornemann, PhD thesis, ETH Zürich, 2020.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2184677, 2184678, 2184711, 2184734 and 2184735. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc03936b |
| This journal is © The Royal Society of Chemistry 2022 |