 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photo-labile BODIPY protecting groups for glycan synthesis†
Sabrina
Leichnitz‡
ab,
Komadhie C.
Dissanayake‡
 c,
Arthur H.
Winter
c,
Arthur H.
Winter
 *c and
Peter H.
Seeberger
*c and
Peter H.
Seeberger
 *ab
*ab
aDepartment of Biomolecular Systems, Max Planck Institute of Colloids and Interfaces, 14476 Potsdam, Germany. E-mail: peter.seeberger@mpikg.mpg.de
bInstitute of Chemistry and Biochemistry, Freie Universität Berlin, 14195 Berlin, Germany
cDepartment of Chemistry, Iowa State University, Ames, Iowa 50014, USA. E-mail: winter@iastate.edu
First published on 1st September 2022
Abstract
Protective groups that can be selectively removed under mild conditions are an essential aspect of carbohydrate chemistry. Groups that can be selectively removed by visible light are particularly attractive because carbohydrates are transparent to visible light. Here, different BODIPY protecting groups were explored for their utility during glycan synthesis. A BODIPY group bearing a boron difluoride unit is stable during glycosylations but can be cleaved with green light as illustrated by the assembly of a trisaccharide.
Carbohydrates are essential in a wide variety of biological processes.1 Rapid access to homogeneous carbohydrate structures provides precious material for immunology, oncology and material science.2 Strategies to chemically synthesize carbohydrates require the orthogonal protection of multiple hydroxyl groups. Protecting group removal mandates often harsh conditions that can result in undesired side reactions. Photo-removable groups offer an attractive tool for the protection of hydroxyls of glycans, as their cleavage is mild and does not require additional reagents, thus avoiding toxic reagents and an additional purification step after deprotection.
The majority of the chromophores of photo-cleavable protecting groups absorb in the ultraviolet region, such as o-nitrobenzyl-,3,4 phenacyl,5 acridinyl,6 benzoinyl,7 and o-hydroxynaphthyl8 structures. Under these conditions, partial deprotection of many other glycan protecting groups, such as benzyl and silyl ethers is possible and photo-deprotection is generally not quantitative. Such chromophores require ultraviolet light that can excite weak UV-absorbing chromophores such as carbonyls, causing side photoreactions. Therefore, visible-light-absorbing photo-cleavable protecting groups are preferred. Recently developed meso-substituted 4,4-disubstituted-4-bora-3a,4a-diaza-s-indacenes (BODIPY) protecting groups9–13 are excellent visible light-sensitive protecting groups that are thermally stable, readily accessible, have tunable absorptions due to many substitution options, and show rapid and efficient release of simple leaving groups. Herein, we developed a concept that allows for the use of these BODIPY protecting groups in glycan synthesis. A series of BODIPY-protected glycan building blocks were prepared and tested in glycosylation reactions for their photochemical lability, and used for oligosaccharide assembly.
The influence of boron substituents on the efficiency of photo-deprotection and glycosylation stability were investigated. Therefore, photo-cleavable BODIPY protected building blocks 1a–c (methyl for 1a, fluoro for 1b, and cyano for 1c) were prepared (Fig. 1).
Since iodonium ions of N-iodosuccinimide (NIS), a commonly used activator in glycan synthesis, reacts with the pyrrolic sides of the BODIPY core by electrophilic aromatic substitution, ethyl- and methyl-groups were incorporated to prevent this side-reaction.
Glycosyl carbonates 1a–b were synthesized using methylimidazolium BODIPY precursors 3a–b and sugar alcohol 2. Moderate yields for the synthesis of 1a–b are not caused by side reactions, as starting materials are recovered after several days of reaction. Heating the reactions lead to decomposition of BODIPY-precursor 3a–b. A comparable imidazolium CN-BODIPY precursor could not be synthesized and strategies including the activation of sugar alcohol 2 using carbonyldiimidazole (CDI) or triphosgene to an alkoxycarbonylimidazole or chloroformate failed. Instead, CN-BODIPY sugar 1c was synthesized by conversion of the fluoro-substituent in 1b by cyanide using TMSCN and SnCl4.
BODIPY protected building blocks 1a–c were tested for photo-deprotection. BODIPY-sugars 1a–c absorb green light (λmax(1b) = 517 nm, λmax(1a) = 538 nm, λmax(1c) = 550 nm, UV-Vis spectra see SI). Optimal photo-deprotection results were achieved in a methanol–chloroform mixture using methanol to enhance the photo-chemical SN1 mechanism10 and chloroform to aid in starting material solubility.
Photo-deprotection was found to be strongly dependent on the substituent at the boron atom (Fig. 2A and B, Me > F > CN). While Me-BODIPY building block 1a was fully deprotected after only ten minutes of green light (525 nm) irradiation, the photolysis of CN-BODIPY building block 1c was significantly slower and not complete after seven hours. The F-BODIPY building block was deprotected after 180 minutes. Though F-BODIPY protected 1b is not as photo-labile as Me-BODIPY building block 1a, it is still acceptable for application in synthesis.
After verifying the photo-lability of synthesized BODIPY-sugars 1a–c, we investigated their applicability for glycan synthesis. To have synthetic utility, not only is an efficient photo-release required for the use of BODIPY-building blocks, but they also need to be stable in the dark under glycosylation conditions. Therefore, BODIPY-sugars were subjected to a glycosylation reaction with C-6 galactose nucleophile 4 (Fig. 2C). BODIPY glycosyl donors carrying electron withdrawing groups at the boron atom - F-BODIPY donor 1b and CN-BODIPY donor 1c – were stable to standard NIS/TfOH glycosylation conditions. On the other hand, Me-BODIPY donor 1a, with the best photo-deprotection results, was not stable under different glycosylation conditions. To avoid decomposition of 1a due to the high acidity of triflic acid, milder TMSOTf was used to promote the leaving group activation in combination with NIS. Buffering the glycosylation mixture using non-nucleophilic base 2,4,6-tri-tert-butylpyrimidine or using an alternative promoter, dimethyl(methylthio)sulfonium trifluoromethanesulfonate (DMTST), did succeed in suppressing the decomposition of 1a.
These results show that the application of photo-labile BODIPY protecting groups to glycan synthesis requires a balance between photo-lability and chemo-stability. Strongly photo-labile Me-BODIPY groups tend to decompose in the presence of catalytic amounts of triflic acid and other promoters, while less photo-labile F- and CN-BODIPY are completely stable under glycosylation conditions and provide high glycosylation yields.
Since the photo-deprotection of CN-BODIPY building block is too slow for a possible application in glycan synthesis and the Me-BODIPY building block is not stable under glycosylation conditions, these two motifs are not applicable for glycan synthesis. However, the protective group on F-BODIPY building block, 1b, can be cleaved in three hours using green light and is a good glycosylating agent (Fig. 2D). Therefore, the F-BODIPY group provides the right balance between glycosylation-stability and photo-reactivity.
Several strategies have been developed in the past to avoid the intermediate purification in the consecutive coupling of building blocks to achieve desired oligosaccharides including AGA,14,15 one-pot strategies,16 poly(tetrafluoroethylene)-assisted purification17 and latent-active glycosylation.18 A recent consecutive glycan assembly approach used photo-labile 2-(2-nitrophenyl)-propyloxycarbonyl protecting groups.19 However, those photo-labile protecting groups require UV light irradiation for photolysis, which creates the risk of partial deprotection of many other protecting groups in synthetic strategies of carbohydrates. The stability of the F-BODIPY protecting group under glycosylation conditions as well as its photo-lability to green light enables consecutive glycan assembly without intermediate purification and selective deprotection. As a proof-of-concept, F-BODIPY building block 1b was employed in the consecutive photo-glycan assembly (Fig. 3) of a trisaccharide. F-BODIPY building block 1b (30 μmol, one equiv.) was glycosylated using acceptor 4 (one equiv.). Quenching with pyridine (0.1 equiv.) and aq. sodium thiosulfate was followed by separation and concentration of the organic phase. The crude residue was then taken up in a chloroform–methanol mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), and subjected to photolysis by green light irradiation for three hours. Disaccharide 5b was directly used upon concentration for the next glycosylation with F-BODIPY building block 1b (one equiv.) to give trisaccharide 6 with a yield of 70% over three steps and only one purification.
3), and subjected to photolysis by green light irradiation for three hours. Disaccharide 5b was directly used upon concentration for the next glycosylation with F-BODIPY building block 1b (one equiv.) to give trisaccharide 6 with a yield of 70% over three steps and only one purification.
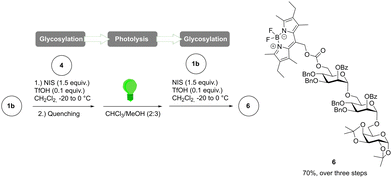 | ||
| Fig. 3 Consecutive assembly of oligosaccharides using F-BODIPY building block 1b and green light for photolysis. | ||
In summary, we demonstrated that photo-labile BODIPY protecting groups can be used for oligosaccharide synthesis. A set of BODIPY-protected carbamate building blocks were synthesized and evaluated for their photo-lability and glycosylation-stability. Of the derivatives tested, F-BODIPY provides the right balance of glycosylation-stability and photo-reactivity. It seems highly likely that other BODIPY-protecting group derivatives can be optimized for even faster photolysis without losing chemostability under glycosylation conditions. Furthermore, because the wavelength of the absorption of the BODIPY-protecting groups can be tuned by various substituents in the range between 450 nm (green) to 700 nm (red)9 another level of orthogonality will be investigated using different BODIPY groups with different absorption, such that deprotection of specific groups may be possible by irradiation with light of different wavelengths (e.g. chromatic orthogonality). For a broader use of BODIPY protecting groups in more challenging glycan syntheses, further studies will be conducted on the solubility in less common solvents, on the stability under other glycosylation conditions as well as under deprotection conditions of other protecting groups. This work provides a new level of orthogonality to glycan synthesis and adds green light as a mild deprotection agent to the set of deprotection strategies in glycan synthesis. Therefore, photo-labile BODIPY groups are promising alternatives to commonly used base-labile 9-fluorenylmethyl carbonate (Fmoc).
S. L. synthesized and characterized the BODIPY protected building blocks and conducted the photolysis and glycosylation experiments. K. C. D. developed, synthesized and characterized the methylimidazolium BODIPY precursors.
The authors acknowledge the Max-Planck Society and the Deutsche Forschungsgemeinschaft (DFG SFB 1449) for generous financial support. A. H. W. acknowledges the NSF (CHE-2055335) for financial support.
Open Access funding provided by the Max Planck Society.
Conflicts of interest
There are no conflicts to declare.Notes and references
- R. A. Dwek, Chem. Rev., 1996, 96, 683–720 CrossRef CAS PubMed.
- M. A. Schmidt, L. W. Riley and I. Benz, Trends Microbiol., 2003, 11, 554–561 CrossRef CAS PubMed.
- J. H. Kaplan and G. Ellis-Davies, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 6571–6575 CrossRef CAS PubMed.
- J. Engels and E. J. Schlaeger, J. Med. Chem., 1977, 20, 907–911 CrossRef CAS PubMed.
- C.-H. Park and R. S. Givens, J. Am. Chem. Soc., 1997, 119, 2453–2463 CrossRef CAS.
- A. J. Ackmann and J. M. Fréchet, Chem. Commun., 1996, 605–606 RSC.
- J. C. Sheehan and R. M. Wilson, J. Am. Chem. Soc., 1964, 86, 5277–5281 CrossRef CAS.
- S. Arumugam and V. V. Popik, J. Am. Chem. Soc., 2009, 131, 11892–11899 CrossRef CAS PubMed.
- J. A. Peterson, C. Wijesooriya, E. J. Gehrmann, K. M. Mahoney, P. P. Goswami, T. R. Albright, A. Syed, A. S. Dutton, E. A. Smith and A. H. Winter, J. Am. Chem. Soc., 2018, 140, 7343–7346 CrossRef CAS PubMed.
- T. Slanina, P. Shrestha, E. Palao, D. Kand, J. A. Peterson, A. S. Dutton, N. Rubinstein, R. Weinstain, A. H. Winter and P. Klán, J. Am. Chem. Soc., 2017, 139, 15168–15175 CrossRef CAS PubMed.
- D. Kand, P. Liu, M. X. Navarro, L. J. Fischer, L. Rousso-Noori, D. Friedmann-Morvinski, A. H. Winter, E. W. Miller and R. Weinstain, J. Am. Chem. Soc., 2020, 142, 4970–4974 CrossRef CAS PubMed.
- P. Shrestha, K. C. Dissanayake, E. J. Gehrmann, C. S. Wijesooriya, A. Mukhopadhyay, E. A. Smith and A. H. Winter, J. Am. Chem. Soc., 2020, 142, 15505–15512 CrossRef CAS PubMed.
- J. A. Peterson, D. Yuan and A. H. Winter, J. Org. Chem., 2021, 86, 9781–9787 CrossRef CAS PubMed.
- H. S. Hahm, M. K. Schlegel, M. Hurevich, S. Eller, F. Schuhmacher, J. Hofmann, K. Pagel and P. H. Seeberger, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E3385 CrossRef CAS.
- J. Danglad-Flores, S. Leichnitz, E. T. Sletten, A. Abragam Joseph, K. Bienert, K. Le Mai Hoang and P. H. Seeberger, J. Am. Chem. Soc., 2021, 143, 8893–8901 CrossRef CAS PubMed.
- S. S. Kulkarni, C.-C. Wang, N. M. Sabbavarapu, A. R. Podilapu, P.-H. Liao and S.-C. Hung, Chem. Rev., 2018, 118, 8025–8104 CrossRef CAS PubMed.
- Y. Feng, J. Wu, G. Chen and Y. Chai, Org. Lett., 2020, 22, 2564–2568 CrossRef CAS PubMed.
- A. Das and N. Jayaraman, Carbohydr. Res., 2021, 508, 108404 CrossRef CAS PubMed.
- J. Wang, Y. Feng, T. Sun, Q. Zhang and Y. Chai, J. Org. Chem., 2022, 87, 3402–3421 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc03851j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |


