Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polyelectrolyte/surfactant films: from 2D to 3D structural control†
Javier
Carrascosa-Tejedor
 ab,
Andreas
Santamaria
ab,
Andreas
Santamaria
 ac,
Andrea
Tummino
ac,
Andrea
Tummino
 a,
Imre
Varga
a,
Imre
Varga
 d,
Marina
Efstratiou
d,
Marina
Efstratiou
 b,
M. Jayne
Lawrence
b,
M. Jayne
Lawrence
 b,
Armando
Maestro
b,
Armando
Maestro
 *ef and
Richard A.
Campbell
*ef and
Richard A.
Campbell
 *b
*b
aInstitut Laue-Langevin, 71 Avenue des Martyrs, CS20156, 38042 Grenoble, France
bDivision of Pharmacy and Optometry, Faculty of Biology, Medicine and Health, University of Manchester, Oxford Road, Manchester M13 9PT, UK. E-mail: richard.campbell@manchester.ac.uk
cDepartamento de Química Física, Facultad de Ciencias, Universidad Complutense de Madrid, Ciudad Universitaria s/n, 28040 Madrid, Spain
dInstitute of Chemistry, Eötvös Loránd University, 112, Budapest H-1518, Hungary
eCentro de Física de Materiales (CSIC, UPV/EHU) - Materials Physics Center MPC, Paseo Manuel de Lardizabal 5, E-20018 San Sebastián, Spain. E-mail: armando.maestro@ehu.eus
fBasque Foundation for Science, Plaza Euskadi 5, Bilbao, 48009, Spain
First published on 31st August 2022
Abstract
Reversible control of the 3D structure of polyelectrolyte/surfactant films at the air/water interface is showcased. A recently discovered mechanism is exploited to form highly efficient, stable and biocompatible films by spreading aggregates composed of poly-L-lysine and sodium dodecyl sulfate on the surface of water. Reversible control of: (1) the surface monolayer coverage, (2) the switching on or off discrete extended structures, and (3) the extended structure coverage is demonstrated for the first time. The intricacy by which the film structures can be controlled is unprecedented and opens exciting potential to optimize film properties by chemical design for novel biomedical transfer applications.
The design of polymer-based nanofilms with controllable architecture attracts strong attention due to applications in areas such as biosensing,1 tissue engineering2 and drug delivery.3 Preparation approaches include Langmuir Blodgett (LB) and Langmuir Schaeffer (LS) deposition onto solids from monolayers at the air/water interface,4 the formation of layer-by-layer (LbL) films on solids,5 and the formation of multilayer structures at both fluid and solid interfaces.6 LB and LS deposition methods benefit from use of a Langmuir trough as the density, morphology and in-plane structures of films can be tuned prior to transfer.7 While a transition from 2D to 3D structures has been reported in surfactant mixtures,8 such an observation has not been made on polymer-based nanofilms to the knowledge of the authors. The development of new film formation methods is therefore of utmost importance.
A new approach to form polyelectrolyte/surfactant (P/S) films at the air/water interface was established a few years ago.9 The approach involves dropping fresh aqueous dispersions of P/S aggregates onto the surface of water, which dissociate and spread material at the surface that remains kinetically trapped in a film due to the entropy associated with counterion release. The aggregates are pre-formed in P/S solutions at a molar ratio where complexes associate due to lack of colloidal stability,10 initially with a size on the order of 100 nm.9 Advantages of the approach include use of water as the spreading solvent and fast creation of films with a high amount of material compared with adsorbed layers, which offer both environmental and economic advantages in potential applications. Recent work showed that poly(sodium styrene sulfonate)/dodecyltrimethylammonium bromide (NaPSS/DTAB) aggregates can nucleate extended structures of different morphologies upon successive spreading or compression of the surface area, A.11 The charge/structure of the aggregates determine if extended structures form, hence an interfacial mechanism where aggregates nucleate self-assembly of extended structures was inferred, conceptually analogous to the function of lung surfactant protein B during respiration.12 Even so, a study limitation was that the extended structures were inferred from a surface excess, Γ, of surfactant exceeding its monolayer coverage, as a direct structural characterization was precluded due to their transient nature.
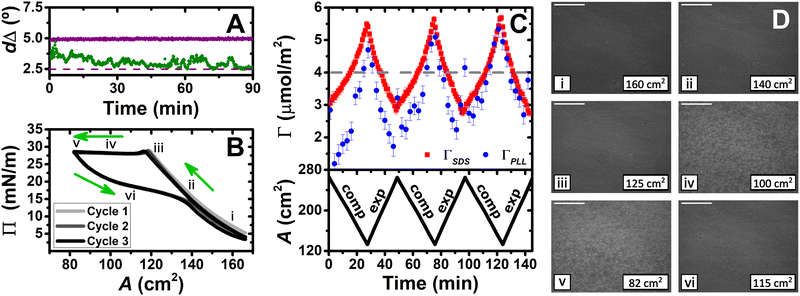
The aim of this work is to control 2D versus 3D structures in P/S spread films of relevance to biomedical transfer applications. The investigated mixture is poly-(L-lysine) (PLL) and sodium dodecyl sulfate (SDS). Both are biocompatible with PLL used in many biomedical applications such as complexing agents with DNA/siRNA for gene delivery,13 and in cancer therapy,14 while SDS is used in oral pharmaceutical formulations up to 1 wt%.15 Also, they are used in film transfer applications such as PLL in a dual-responsive nanofluidic device,16 to promote cellular adhesion,17 and for skin regeneration,18 and SDS in self-healing hydrogels.19 An important pre-requisite of studying this system was that extended structures formed upon film compression are stable with time, allowing them to be characterized directly, and making them robust to potential applications. PLL was chosen over other polypeptides because in the bulk SDS can induce rigid in-plane interactions in a β-sheet conformation.20 This pre-requisite was met, as ellipsometry data comparing compressed spread films of the NaPSS/DTAB and PLL/SDS systems show that only the latter type of film is stable (Fig. 1A).
Our methodology is to form a film by dispensing aqueous droplets containing PLL/SDS aggregates onto the water surface of a Langmuir trough and use barriers to control its surface area. The spreading solutions comprise 100 ppm PLL and 0.80 mM SDS, which self-assemble to form negatively charged aggregates as characterized by zeta potential (ESI,† Section S1), chosen simply because aggregates charged with excess surfactant are effective in forming extended structures for the NaPSS/DTAB system.11 Several reflectometry techniques are applied to complement in situ measurements of the surface pressure, Π. Ellipsometry is used to measure the phase shift from the presence of the film, dΔ, which is related to the total amount of interfacial material per unit area.21 Brewster angle microscopy (BAM) is used to image the lateral morphology on the μm-scale.22 Three implementations of neutron reflectometry (NR) are applied to resolve the film composition and structure. First is the recently-developed low-Qz analysis (0.01–0.03 Å−1), where Qz is the momentum transfer normal to the interface, to resolve the surface excesses of PLL and SDS during three compression/expansion cycles.23 Second is the full-Qz analysis (0.01–0.25 Å−1) to resolve the interfacial structure at a high film compression state.24 Third is a novel mid-Qz analysis (0.02–0.12 Å−1) to resolve the structural dynamics during one cycle. Full details about the materials and methods used are provided (ESI,† Section S2).
The dynamics of PLL/SDS spread films were first examined by combining measurements of Π with the low-Qz analysis of NR, ellipsometry and BAM. First, the Π–A film response over three cycles is shown (Fig. 1B). Film compression initially results in a continuous increase in Π, attributed to enhanced molecular interactions with an increasing number of surfactant molecules per unit area in what we term the ‘surface monolayer’, i.e., a layer of surfactant in contact with air (hydrophobic driving force) with hydrated polyelectrolyte bound to the headgroups (electrostatic driving force).25 Upon further compression, beyond state iii, Π remains constant at ∼28 mN m−1, which is attributed to collapse of the surface monolayer, i.e. expulsion of material either to bound extended structures or the bulk. Expansion of the film is characterized by a large hysteresis in Π, the presence of a pseudo-plateau and a final value of Π close to the initial one, consistent with reincorporation of material back into the surface monolayer yet with a kinetic barrier.
The low-Qz analysis of NR was applied to provide the surface excesses of PLL and SDS (Fig. 1C). An explanation of the fitting procedure is provided (ESI,† Section S3). In comparison with the value ΓSDS = 4.0 ± 0.1 μmol m−2 at the collapse, upon further compression ΓSDS continually rises to exceed this value. This observation demonstrates that the material expelled from the surface monolayer beyond the collapse remains bound in the form of extended structures rather than being lost to the bulk. From ΓSDS = 5.5 ± 0.1 μmol m−2 at the maximum compression state measured, and with the assumption that ΓSDS in the surface monolayer does not increase by further compression of the film after the collapse, it may be noted that 27 ± 1% of the SDS would be in the extended structures; we will return to this point later. Moreover, despite the large hysteresis in Π, there is no hysteresis in Γ, indicating that there are equivalent amounts of material in the film at corresponding surface areas during compression or expansion, which suggests that the hysteresis is attributed to a kinetic barrier for the transfer of material from the extended structures to the surface monolayer. These results are supported qualitatively in values of dΔ recorded over successive cycles using ellipsometry (ESI,† Section S4). Lastly, the film composition over successive cycles is generally similar, hinting at their robustness to potential applications.
Next, we applied BAM to visualize the films (Fig. 1D). While prior to the collapse, images i, ii and iii are uniform with increasing brightness, images iv and v after exhibit discrete regions on the μm-scale that grow in number during compression, which we have reproduced in higher resolution combined with analysis of their coverage (ESI,† Section S5). The regions are attributed to discrete extended structures in contact with the surface monolayer and disappear in image vi upon expansion due to transfer of material back to the surface monolayer resulting in a homogeneous film once again.
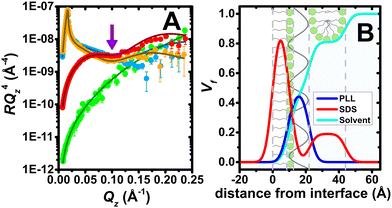
A second implementation of NR exploiting the full Qz-range allows us to resolve the extended structures of PLL/SDS films at maximum compression state v (Fig. 1B). Neutron reflectivity profiles in 4 isotopic contrasts with fits to a common interfacial model in stratified layers (Fig. 2A), and volume fraction (Vf) profiles (Fig. 2B) are shown. An explanation of the fitting procedure and a table of structural parameters is provided (ESI,† Section S6). A Kiessig fringe observed in data in the two isotopic contrasts that have the greatest scattering contrast between the surfactant chains and solvent, i.e., PLL with deuterated SDS in air contrast matched water (ACMW, 8.1% v/v D2O in H2O) and PLL with SDS in D2O (purple arrow), reveal the presence of extended structures with surfactant present below the surface monolayer. The extended structure layer has a thickness of 21.8 ± 0.8 Å (almost twice that of the SDS monolayer), and a model also involving PLL in the layer did not improve the fit. We infer that the structures are either discrete patches of SDS bilayer wrapped by unresolved PLL or bound SDS hemimicelles. The amount of SDS present in the extended structure is 29 ± 1%, consistent with the 27 ± 1% calculated above on the assumption that the surface monolayer does not change in coverage beyond the collapse. Although extended structures in adsorbed P/S layers have been recently the focus of an experimental study,26 here we have resolved for the first time the extended structures of a P/S film formed by the aggregate spreading approach.
Lastly, we performed a third implementation of NR involving a novel mid-Qz analysis to follow the structural dynamics of PLL/SDS films during one cycle. A single contrast of PLL with SDS in D2O was chosen for maximum sensitivity to the surfactant in the extended structure (strong difference in scattering between the chains and solvent) compared with in the surface monolayer (weak difference in scattering between the chains and air). The scope is to resolve the change in thickness or coverage of the extended structures with respect to film compression. Data until the collapse (states i, ii and iii in Fig. 1B), combined with simulations of the reflectivity profiles based on the coverage of the surface monolayer from Fig. 1C, show that changing monolayer coverage indeed has a small effect on the data (Fig. 3A). However, data from the collapse (states iii, iv and v), combined with fits of the volume fraction at a constant extended structure thickness of 21.8 Å reveal the higher sensitivity of the measurements to the coverage of the extended structure (Fig. 3B). By comparison, fits of the data after the collapse to a model where the thickness of the extended structure changes at a constant volume fraction exhibited significantly worse agreement, as revealed by an increase of up to 30% in the global χ2 parameter (ESI,† Section S7). It can be concluded that after collapse of the surface monolayer, the coverage of discrete patches of the wrapped bilayer or hemimicelles can now be controlled.
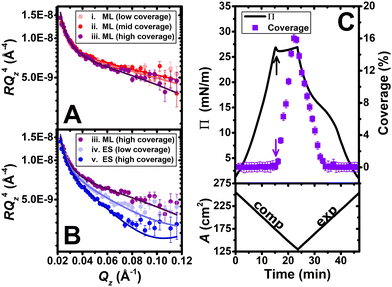 | ||
| Fig. 3 Neutron reflectivity profiles of a PLL/SDS spread film in D2O at different compression states: (A) monolayer (ML) region and (B) extended structure (ES) region; states i–v are defined in Fig. 1B; solid lines show the simulated ML models and fitted ES models. (C) Variation of Π (black line) and fitted extended structure coverage (violet squares) with respect to the time; black and violet arrows indicate film collapse and the onset of extended structures, respectively; variation of area versus time is also shown. | ||
The coverage of extended structures, in comparison with the variation of Π, as a function of time is displayed (Fig. 3C). The extended structures start to form (violet arrow) at the collapse (black arrow). The coverage increases with film compression beyond the collapse and decreases back down to 0% during the Π pseudo-plateau upon expansion, indicating that the surfactant in the extended structures has been fully resupplied to the monolayer. While the increase in coverage of extended structures takes ∼7 min, the reduction takes ∼10 min, consistent with the kinetic barrier to resupply of material to the surface monolayer. Although NR has been used previously to resolve changes in film composition under surface area dynamic,9,11 this is the first time to the knowledge of the authors that it has been used to resolve changes in the extended structures of a dynamic air/water interface, and critically it highlights the unprecedented control gained over 2D versus 3D structures in P/S films, schematically illustrated (Fig. 4).
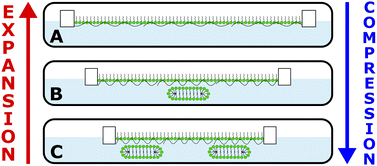 | ||
| Fig. 4 Schematic illustration of (A) control of the coverage of the surface monolayer, (B) switching on or off 3D extended structures, and (C) control of the coverage of 3D extended structures. | ||
The PLL/SDS spread films studied in the present work are stable and reversible in their dynamics, which make them robust and amenable to transfer applications.27 The combination of the new film spreading approach with established deposition techniques may offer economic and environmental advantages as the amount of materials used and waste generated are low compared to other methods.28 As a result, 3D structures could be designed with controllable architecture and deposited onto solids for biomedical applications such as wound dressings, antimicrobial coatings or drug delivery applications.29
Coupling of reflectometry techniques to a Langmuir trough has allowed us to elucidate the compositional and structural dynamics and directly characterize the extended structures in P/S spread films for the first time. This approach must now be urgently applied to other systems to understand in greater depth the physicochemical film properties, such as by tuning specific amino acid interactions with reference to different polypeptide conformations adopted in the bulk (e.g. α-helices by polyarginine/SDS versus β-sheets by PLL/SDS).20
The mid-Qz structural dynamic application of NR showcased in this study opens the possibility to understand a wide variety of synthetic and biological systems that adopt 3D morphologies. Further applications of the method include, for example, particle–laden interfaces that present buckling or jamming phenomena,30 and lung surfactant models where the transfer of lipid between the surface monolayer and extended structures during respiration is vital for the stabilization of alveoli.12
We thank the Institut Laue-Langevin for beam time on FIGARO (DOIs: https://doi.org/10.5291/ILL-DATA.9-12-614 and https://doi.org/10.5291/ILL-DATA.9-12-631), Simon Wood for technical assistance and the Partnership for Soft Condensed Matter (PSCM) for lab support. IV acknowledges the financial support from the Hungarian National Research, Development and Innovation Office (NKFIH K116629). AM acknowledges the financial support from MICINN under grant PID2021-129054NA-I00 and the IKUR Strategy of the Basque Government.
Conflicts of interest
There are no conflicts of interest to declare.References
- W. Guan, W. Zhou, J. Lu and C. Lu, Chem. Soc. Rev., 2015, 44, 6981 RSC.
- P. T. Hammond, Mater. Today, 2012, 15, 196 CrossRef CAS.
- A. N. Zelikin, ACS Nano, 2010, 4, 2494 CrossRef CAS PubMed.
- O. N. Oliveira, L. Caseli and K. Ariga, Chem. Rev., 2022, 122, 6459 CrossRef PubMed.
- J. J. Richardson, J. Cui, M. Björnmalm, J. A. Braunger, H. Ejima and F. Caruso, Chem. Rev., 2016, 116, 14828 CrossRef CAS PubMed.
- R. A. Campbell, M. Yanez Arteta, A. Angus-Smyth, T. Nylander and I. Varga, J. Phys. Chem. B, 2012, 116, 7981 CrossRef CAS PubMed.
- K. Ariga, Langmuir, 2020, 36, 7158 CrossRef CAS PubMed.
- C. Rubia-Payá, E. Jimenez-Millán, J. J. Giner-Casares, G. Brezesinski, M. T. Martín-Romero and L. Camacho, Langmuir, 2013, 29, 4796 CrossRef PubMed.
- R. A. Campbell, A. Tummino, B. A. Noskov and I. Varga, Soft Matter, 2016, 12, 5304 RSC.
- R. Mészáros, L. Thompson, M. Bos, I. Varga and T. Gilányi, Langmuir, 2003, 19, 609 CrossRef.
- A. Tummino, J. Toscano, F. Sebastiani, B. A. Noskov, I. Varga and R. A. Campbell, Langmuir, 2018, 34, 2312 CrossRef CAS PubMed.
- J. C. Castillo-Sánchez, A. Cruz and J. Pérez-Gil, Arch. Biochem. Biophys., 2021, 703, 108850 CrossRef PubMed.
- B. Shi, M. Zheng, W. Tao, R. Chung, D. Jin, D. Ghaffari and O. C. Farokhzad, Biomacromolecules, 2017, 18, 2231 CrossRef CAS PubMed.
- N. Li, L. Zhao, L. Qi, Z. Li and Y. Luan, Prog. Polym. Sci., 2016, 58, 1 CrossRef CAS.
- P. Y. Lin, E. Y. Chuang, Y. H. Chiu, H. L. Chen, K. J. Lin, J. H. Juang, C. H. Chiang, F. L. Mi and H. W. Sung, J. Controlled Release, 2017, 259, 168 CrossRef CAS PubMed.
- J. Li, P. An, C. Qin, C. L. Sun, M. Sun, Z. Ji, C. Wang, G. Du, J. Liu and Y. Xie, ACS Omega, 2020, 5, 4501 CrossRef CAS PubMed.
- P. P. Pokharna, M. K. Ghantasala and E. A. Rozhkova, Mater. Today Commun., 2021, 27, 102348 CrossRef CAS.
- J. Lam, E. C. Clark, E. L. S. Fong, E. J. Lee, S. Lu, Y. Tabata and A. G. Mikos, Biomaterials, 2016, 83, 332 CrossRef CAS PubMed.
- D. C. Tuncaboylu, M. Sari, W. Oppermann and O. Okay, Macromolecules, 2011, 44, 4997 CrossRef CAS.
- P. Novotná and M. Urbanová, Anal. Biochem., 2012, 427, 211 CrossRef PubMed.
- J. A. De Feijter, J. Benjamins and F. A. Veer, Biopolymers, 1978, 17, 1759 CrossRef CAS.
- W. Daear, M. Mahadeo and E. J. Prenner, Biochim. Biophys. Acta, Biomembr., 2017, 1859, 1749 CrossRef CAS PubMed.
- R. A. Campbell, Curr. Opin. Colloid Interface Sci., 2018, 37, 49 CrossRef CAS.
- M. W. A. Skoda, Curr. Opin. Colloid Interface Sci., 2019, 42, 41 CrossRef CAS.
- M. Uhlig, O. Löhmann, S. Vargas Ruiz, I. Varga, R. von Klitzing and R. A. Campbell, Chem. Commun., 2020, 56, 952 RSC.
- L. Braun, M. Uhlig, O. Löhmann, R. A. Campbell, E. Schneck and R. von Klitzing, ACS Appl. Mater. Interfaces, 2022, 14, 27347 CrossRef CAS PubMed.
- Y.-L. Lee, A. Dudek, T. N. Ke, F. W. Hsiao and C. H. Chang, Macromolecules, 2008, 41, 5845 CrossRef CAS.
- J. Lipton, G. M. Weng, J. A. Röhr, H. Wang and A. D. Taylor, Matter, 2020, 2, 1148 CrossRef.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101 CrossRef.
- B. D. Leahy, L. Pocivavsek, M. Meron, K. L. Lam, D. Salas, P. J. Viccaro, K. Y. C. Lee and B. Lin, Phys. Rev. Lett., 2010, 105, 058301 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: (1) Zeta potential measurements of PLL/SDS aggregates, (2) materials and methods, (3) low-Qz compositional analysis: fitting, (4) ellipsometry data on PLL/SDS spread film dynamics, (5) BAM images, (6) full-Qz structural analysis: fitting and parameters, and (7) mid-Qz structural dynamics analysis: fitting. See DOI: https://doi.org/10.1039/d2cc03766a |
| This journal is © The Royal Society of Chemistry 2022 |